Slow-release preparation of ticagrelor
A slow-release preparation, the technology of ticagrelor, is used in medical preparations containing active ingredients, pill delivery, cardiovascular system diseases, etc., which can solve problems such as waste and achieve the effect of avoiding waste.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
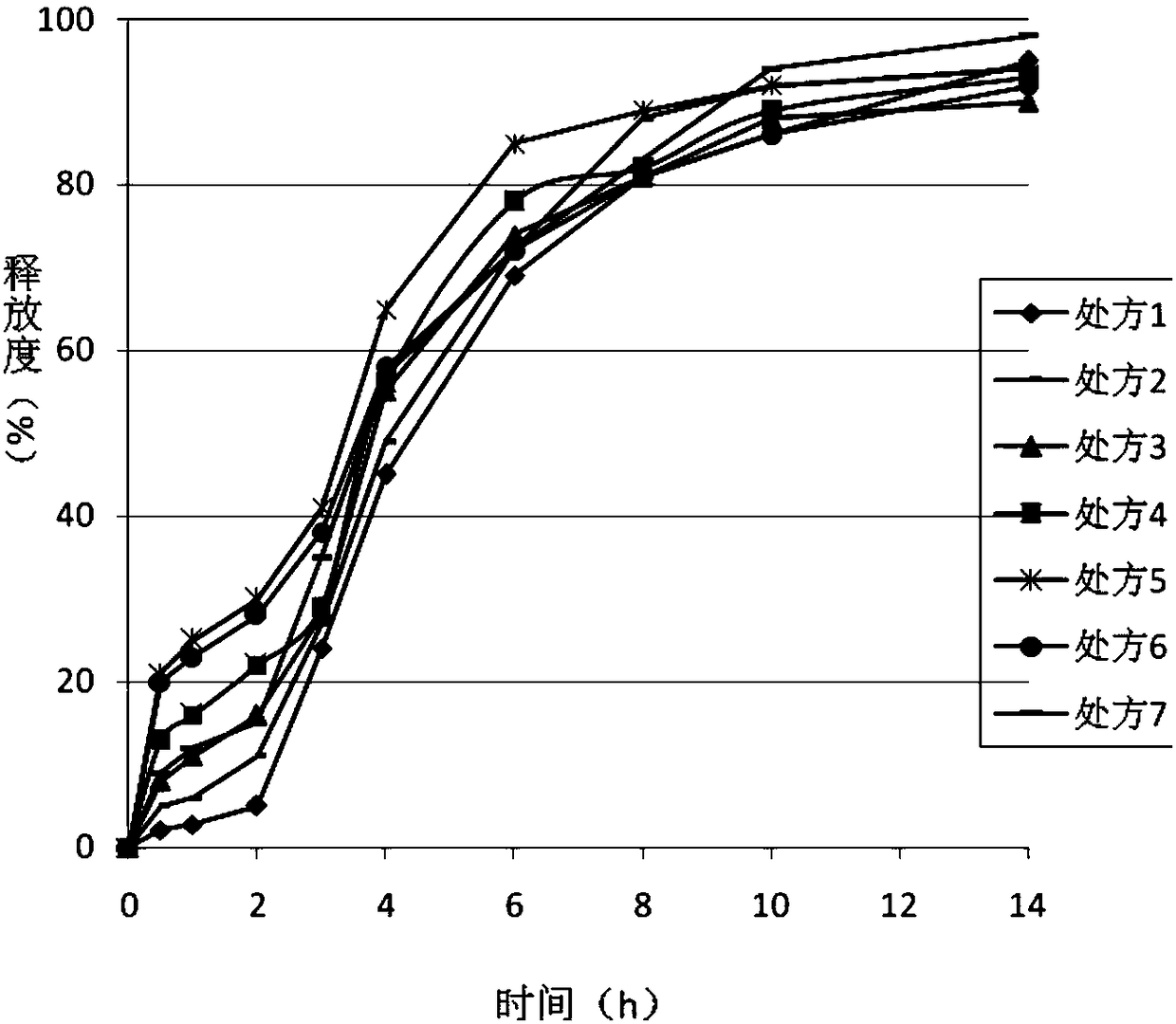
[0042] Premix ticagrelor with the materials in the prescription, add binders for wet granulation, add magnesium stearate to mix the granules evenly, and then compress and coat the granules to prepare immediate-release tablet cores; according to the prescription information, The immediate-release tablet core is coated with sustained-release material, enteric-coated material and drug-containing layer in turn, and dried. The release of the prepared formulations was evaluated.
[0043]
[0044]
[0045] The chromatographic conditions for the determination of the release rate are: use octadecylsilane bonded silica gel as the chromatographic column filler, use 10ml of phosphate buffer [take 1.0mol / L sodium dihydrogen phosphate solution (adjust the pH value to 3.0 with phosphoric acid), Add water to 480ml, shake well]-acetonitrile (48:52) as mobile phase; column temperature is 55°C; detection wavelength is 242nm. The tailing factor of the ticagrelor peak should not be greater ...
Embodiment 2
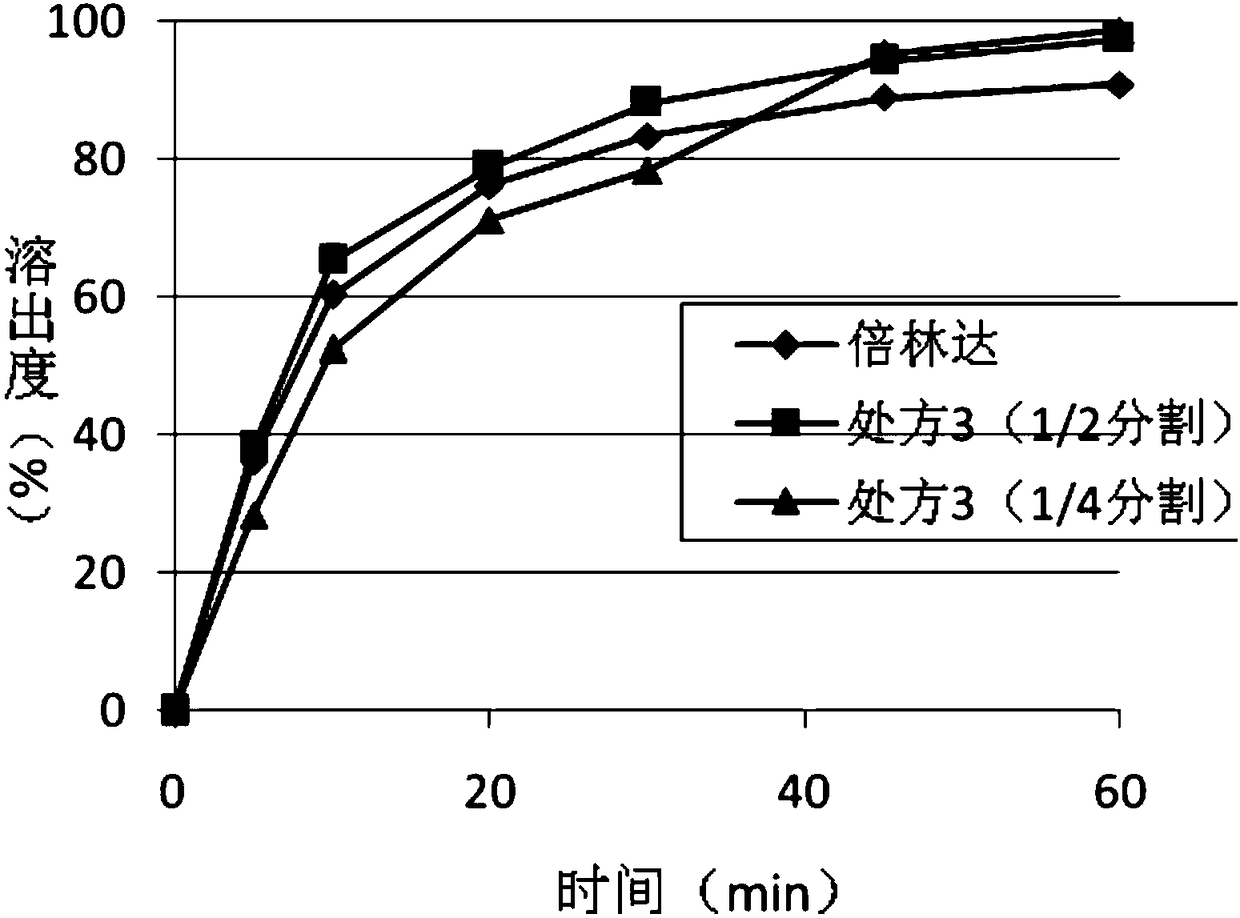
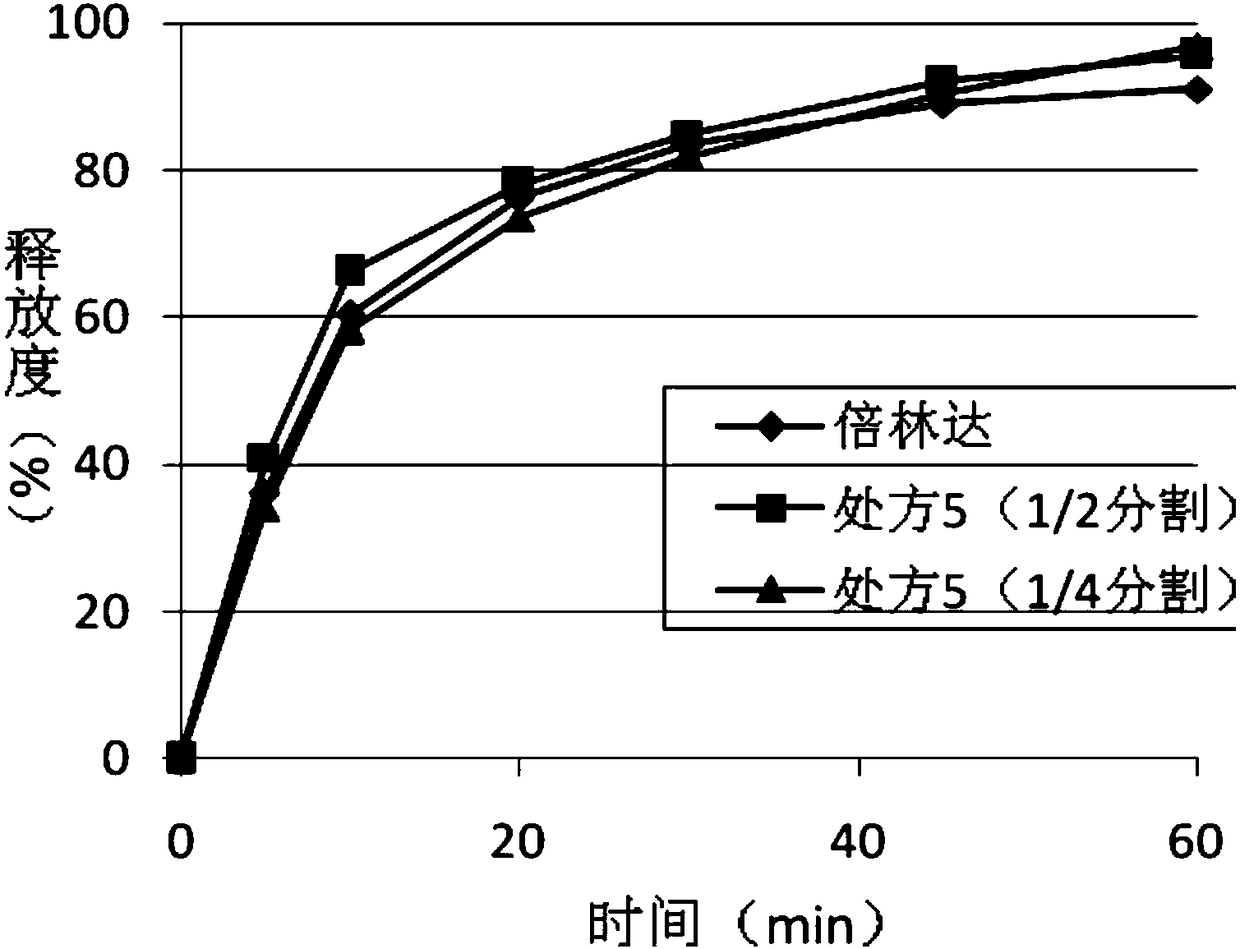
[0047] The tablets of prescription 3 and prescription 5 in the embodiment are cut according to the form of 1:1 and 1:3, that is, split into 1 / 2 and 1 / 4. Put all parts of the cut tablet into the dissolution cup, and investigate the dissolution behavior in the dissolution medium of 0.1NHCL with 0.2% Tween 80 at the same time as the commercially available 2 tablets of Belinda, and the dissolution curve figure 2 with image 3 .
[0048] According to the above data, it is shown that the ticagrelor film-controlled sustained-release tablet of the present invention can achieve a certain release in the early stage and a stable release in the later stage in the completed state. The tablet of the present invention has an in vitro dissolution behavior similar to that of the original commercially available immediate-release tablet after cutting and breaking the coating film at different ratios. Therefore, the preparation of the present invention can not only meet the requirement of once...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com