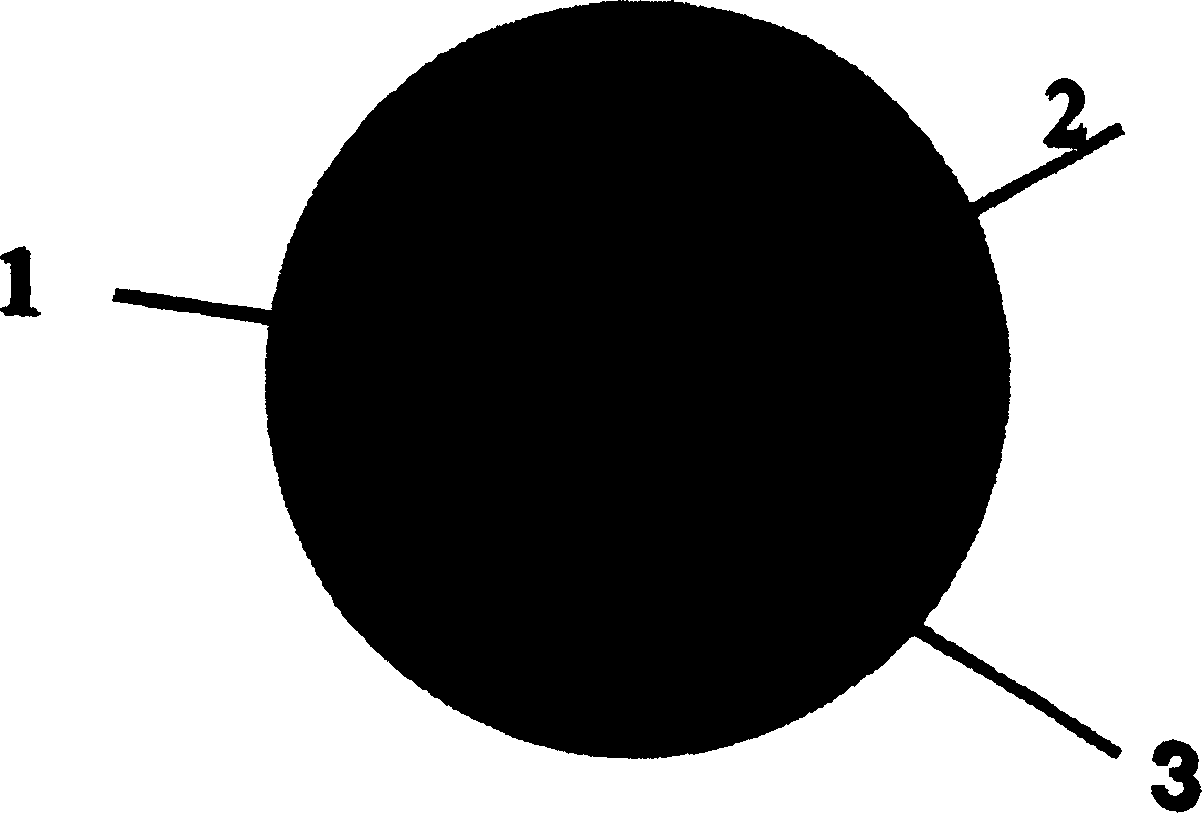
Preparation method of medicine elution type blood vessel stent
A technology of vascular stents and drugs, which is applied in the direction of blood vessels, stents, and devices with tubular structures in the human body, can solve the problems of easy detachment of the polymer layer, achieve the effect of not easy detachment, and overcome the inability to be co-soluble in the same volatile organic solvent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A kind of operation step of preparation method of hirudin-eluting vascular stent is as follows:
[0040] (1) Preparation of butyl titanate solution
[0041]The butyl titanate solution is prepared according to the following substances and molar concentration ratios, butyl titanate: ethylene glycol methyl ether: ethyl acetoacetate: water = 0.5: 10: 0.5: 1, first add titanium to ethylene glycol methyl ether butyl ester, mix well and then add ethyl acetoacetate and water;
[0042] (2) Place the vascular stent in butyl titanate solution for processing
[0043] Soak the vascular stent in butyl titanate solution for 5 minutes, take it out and dry it at room temperature, then place it in a muffle furnace for heat treatment at 600°C, the heating rate is 8°C / min, keep it warm for 5 hours, take it out and dry it at room temperature cooling the vascular stent to form a titanium dioxide layer with a surface roughness of 0.5 μm on the surface of the vascular stent;
[0044] (3) Pr...
Embodiment 2
[0050] The operation steps of a preparation method of an anti-platelet monoclonal antibody eluting vascular stent are as follows:
[0051] (1) Preparation of butyl titanate solution
[0052] The butyl titanate solution is prepared according to the following substances and molar concentration ratios, butyl titanate: ethylene glycol methyl ether: ethyl acetoacetate: water = 3: 30: 3: 10, first add titanium to ethylene glycol methyl ether butyl ester, mix well and then add ethyl acetoacetate and water;
[0053] (2) Place the vascular stent in butyl titanate solution for processing
[0054] Soak the vascular stent in butyl titanate solution for 5 minutes, take it out, dry it at room temperature, and then place it in a muffle furnace for heat treatment at 450°C at a heating rate of 4°C / min, keep it warm for 0.5 hours, take out the stent, A titanium dioxide layer with a surface roughness of 0.1 μm is formed on the surface of the vascular stent;
[0055] (3) Preparation of polymer...
Embodiment 3
[0061] The operation steps of a method for preparing a protamine-eluting vascular stent are as follows:
[0062] (1) Preparation of butyl titanate solution
[0063] The butyl titanate solution is prepared according to the following substances and molar concentration ratios, butyl titanate: ethylene glycol methyl ether: ethyl acetoacetate: water = 2: 20: 2: 5, first add titanium to ethylene glycol methyl ether butyl ester, mix well and then add ethyl acetoacetate and water;
[0064] (2) Place the vascular stent in butyl titanate solution for processing
[0065] Soak the vascular stent in butyl titanate solution for 20 minutes, take it out and dry it at room temperature, then place it in a muffle furnace for heat treatment at 550°C at a heating rate of 6°C / min, keep it warm for 3 hours, take it out and dry it at room temperature cooling the vascular stent to form a titanium dioxide layer with a surface roughness of 0.3 μm on the surface of the vascular stent;
[0066] (3) Pre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface roughness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| surface roughness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com