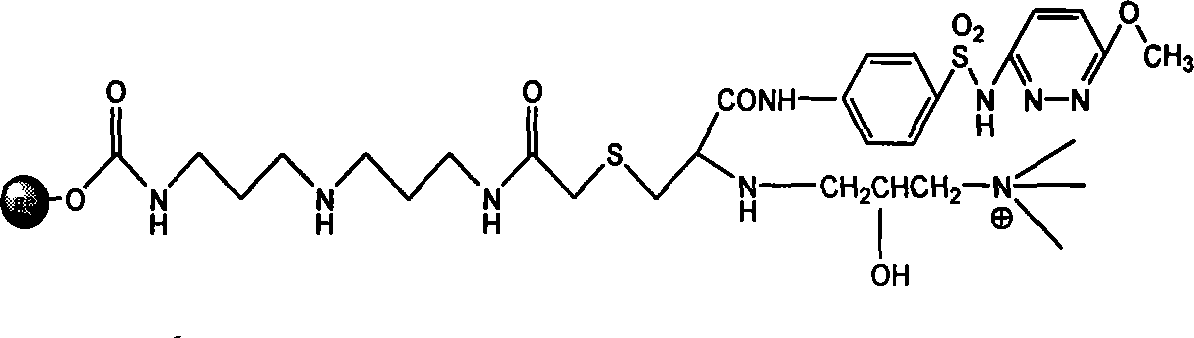
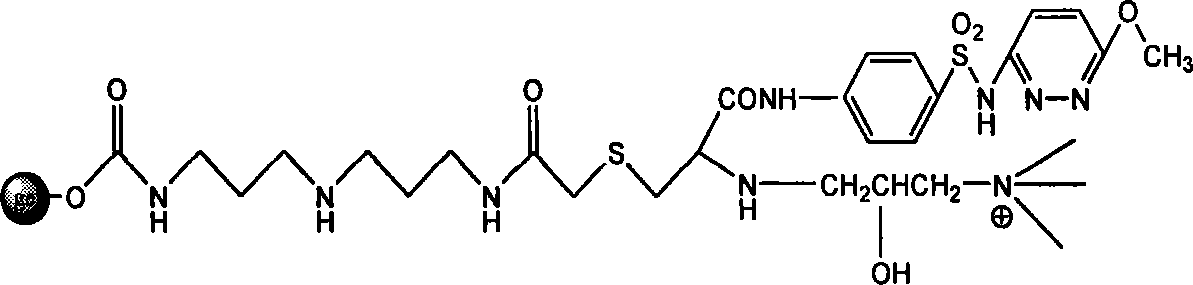
Endotoxin adsorption material for curing endotoxemia
An endotoxemia and adsorption material technology, applied in the fields of biological separation and biomedical engineering, can solve the problems of antibody source, preparation and purification difficulties, neurotoxicity and nephrotoxicity, unsuitable for removing endotoxin, etc. Improved adsorption selectivity, wide-ranging and inexpensive effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the preparation of adsorption material
[0035] Step 1: Activation reaction of agarose gel
[0036] Take 100 mL of agarose gel, filter it with deionized water 4-5 times to remove the preservative in the gel, and wash it successively with 30%, 70%, 100% (v / v) acetone aqueous solution of 5 times the gel volume Gel, then put the washed gel in 100ml acetone, add 10g N,N'carbonyldiimidazole (CDI), and react in a shaker at 30°C and 150 rpm for 2 hours. After the reaction was completed, suction filtered, and the activated gel was repeatedly washed with acetone 5 times the volume of the gel to obtain activated agarose gel.
[0037] Step 2: Coupling the spacer arm
[0038] Put 100ml of activated agarose gel in 100ml of acetone, add 10mL of bisamino reagent (diaminodipropylimine), and react in a shaker at 30°C and 150 rpm for 2 hours. Suction filtration after the reaction finishes, wash the gel after the reaction repeatedly with deionized water of 5 times the gel v...
Embodiment 2
[0042] Embodiment 2: heat source removal treatment of adsorption material and vessel
[0043] The glass instruments touched by the experiment were dry baked at 300°C for 3 hours to remove the heat source. Plastic containers at 30% H 2 o 2 Soak overnight, then rinse with heat-free water and allow to dry. The prepared adsorbent material was first soaked in 1.5M NaOH solution overnight, then placed in a perfusion column, rinsed with 0.2M NaOH (containing 20% ethanol) at 2ml / min for 1 hour, and finally rinsed with normal saline and pyrogen-free water respectively Rinse at 2ml / min for 1 hour to obtain the adsorption material for sterilization and heat removal.
Embodiment 3
[0044] Embodiment 3: Dynamic adsorption removes endotoxin in human plasma system
[0045] Take 2ml of the adsorbent material and put it into the chromatographic column, control the flow rate of 10ml / min by a constant flow pump at room temperature, perfuse with 10ml of human plasma containing endotoxin for 2 hours, take the supernatant to measure the concentration of each component. (Table 1)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com