Ticagrelor oral-disintegrating sustained release tablet and preparation method thereof
A technology of ticagrelor and sustained-release tablets, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problem of patients missing medication, increasing the risk of acute thrombosis, and unfavorable patients problems swallowing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Preparation of orally disintegrating sustained-release tablets of ticagrelor
[0022] Prescription composition:
[0023]
[0024]
[0025] Preparation Process:
[0026] (1) Co-dissolve ticagrelor and ethyl cellulose in ethyl acetate to form an oil phase, dissolve sodium lauryl sulfate in a saturated aqueous solution of ethyl acetate to form a water phase, slowly drop the oil phase into water Microspheres are formed in the phase, and then add an appropriate amount of water to precipitate the microspheres, wash with pure water three times, and dry at 60°C to constant weight to obtain the microspheres;
[0027] (2) Weigh ticagrelor sustained-release microspheres, microcrystalline cellulose PH101 and low-substituted hydroxypropyl cellulose, mix evenly, granulate with purified water, granulate with 24 mesh, dry the granules at 50°C to 1~ 3%, 24 mesh whole grains, plus magnesium stearate and aspartame, compressed into tablets. The ticagrelor orally disinte...
Embodiment 2
[0028] Example 2: Preparation of orally disintegrating sustained-release tablets of ticagrelor
[0029] Prescription composition:
[0030]
[0031]
[0032] Preparation Process:
[0033] (1) Dissolve ticagrelor and polylactic acid in ethyl acetate to form an oil phase, dissolve poloxamer 188 in a saturated aqueous solution of ethyl acetate to form a water phase, slowly drop the oil phase into the water phase to form Microspheres, then add appropriate amount of water to precipitate the microspheres, wash with pure water three times, and dry in a fluidized bed to constant weight to obtain the microspheres;
[0034] (2) Weigh ticagrelor sustained-release microspheres, maltodextrin, hydroxypropyl methylcellulose and crospovidone, mix evenly, use purified water to granulate, granulate with 24 mesh, and dry the granule moisture at 50°C To 1~3%, 24 mesh whole grains, plus talcum powder, magnesium stearate and sucralose, compressed into tablets. The orally disintegrating sust...
Embodiment 3
[0035] Example 3: Preparation of orally disintegrating sustained-release tablets of ticagrelor
[0036] Prescription composition:
[0037]
[0038]
[0039] Preparation Process:
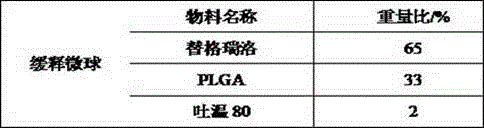
[0040] (1) Dissolve ticagrelor and PLGA in ethyl acetate to form an oil phase, dissolve Tween 80 in a saturated aqueous solution of ethyl acetate to form a water phase, slowly drop the oil phase into the water phase to form microspheres, Then add an appropriate amount of water to precipitate the microspheres, wash with pure water three times, and dry in a fluidized bed to constant weight to obtain the microspheres;
[0041] (2) Weigh ticagrelor sustained-release microspheres, mannitol, microcrystalline cellulose PH101 and pregelatinized starch, granulate with purified water, granulate with 24 mesh, and dry the granules at 50°C to 1-3% moisture , 24 mesh whole grains, plus magnesium stearate and stevioside, compressed into tablets. The orally disintegrating sustained-release tablet of ticagre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com