Capecitabine granule and preparation method thereof
A technology of capecitabine and granules, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
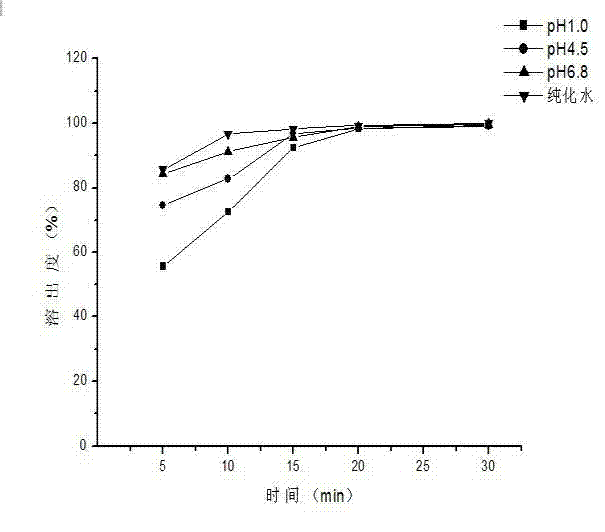
Embodiment 1
[0043]The prescription composition of capecitabine granules: the specification is 0.5g (calculated as capecitabine), and the prescription quantity is 1000 bags.
[0044]
[0045] Preparation Process:
[0046] 1) Pass capecitabine, β-cyclodextrin and auxiliary materials through an 80-mesh sieve for later use; take the prescribed amount of hydroxypropyl methylcellulose, add an appropriate amount of water to prepare a 2% hydroxypropyl methylcellulose aqueous solution, and set aside;
[0047] 2) Weigh the cyclodextrin according to the prescription amount to make a saturated aqueous solution at 50°C, then place it in a high-shear wet granulator, start stirring, and the stirring speed is 600rpm / min;
[0048] 3) Weigh capecitabine according to the prescription amount and add it to the above solution, stir at constant temperature for 45 minutes, stop heating and continue stirring to room temperature, stop stirring after 3 hours, spray dry, and obtain cyclodextrin inclusion compound...
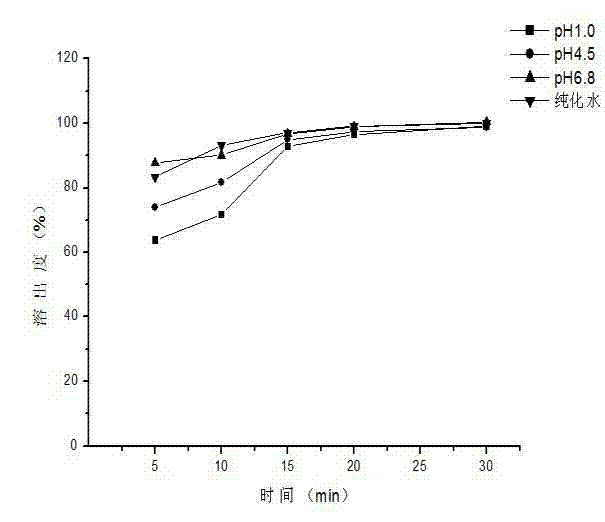
Embodiment 2
[0051] The prescription composition of capecitabine granules: the specification is 0.5g (calculated as capecitabine), and the prescription quantity is 1000 bags.
[0052]
[0053] Preparation Process:
[0054] 1) Pass capecitabine, hydroxy-β-cyclodextrin and auxiliary materials through an 80-mesh sieve for later use; take the prescribed amount of hydroxypropyl cellulose, add appropriate amount of water to prepare a 5% aqueous solution of hydroxypropyl cellulose for later use;
[0055] 2) Weigh hydroxy β-cyclodextrin according to the prescription amount to make a saturated aqueous solution at 60°C, then place it in a high-shear wet granulator, start stirring, and the stirring speed is 800rpm / min;
[0056] 3) Weigh capecitabine according to the prescription amount and add it to the above solution, stir at constant temperature for 45 minutes, stop heating and continue stirring to room temperature, stop stirring after 5 hours, spray dry, and obtain cyclodextrin inclusion compou...
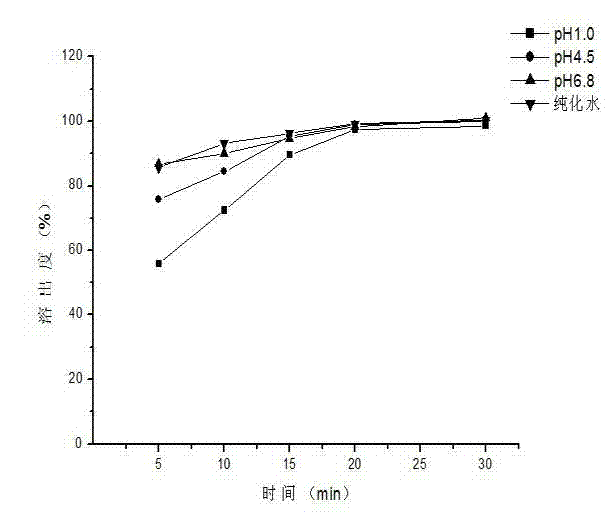
Embodiment 3
[0059] The prescription composition of capecitabine granules: the specification is 0.5g (calculated as capecitabine), and the prescription quantity is 1000 bags.
[0060]
[0061] Preparation Process:
[0062] 1) Pass capecitabine, gamma-cyclodextrin and auxiliary materials through an 80-mesh sieve for subsequent use; take the povidone of the prescribed amount, add appropriate amount of water to prepare a 4% aqueous solution of povidone for subsequent use;
[0063] 2) Weigh γ-cyclodextrin according to the prescription amount to make a saturated aqueous solution at 55°C, then place it in a high-shear wet granulator, start stirring, and the stirring speed is 400rpm / min;
[0064] 3) Weigh capecitabine according to the prescription amount and add it to the above solution, stir at constant temperature for 45 minutes, stop heating and continue stirring to room temperature, stop stirring after 4 hours, spray dry, and obtain cyclodextrin inclusion compound;
[0065] 4) Pass the pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com