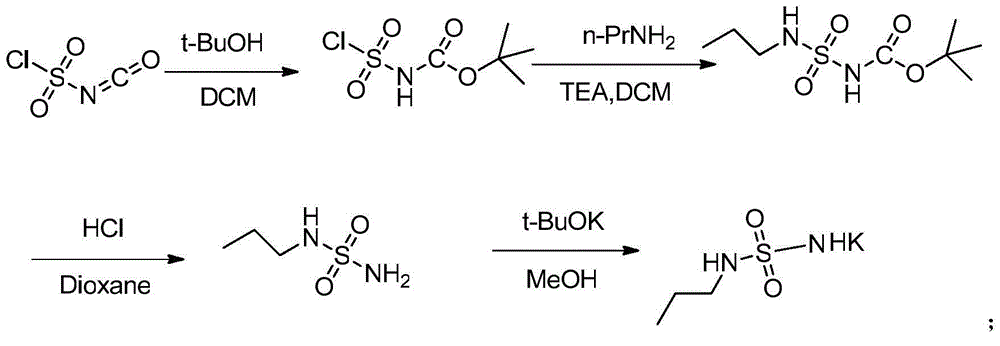
Method for preparing macitentan
A technology of macitentan and compounds, applied in the field of preparation of macitentan, can solve problems such as uneconomical and increased production costs, and achieve the effects of low production costs, cheap raw materials, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
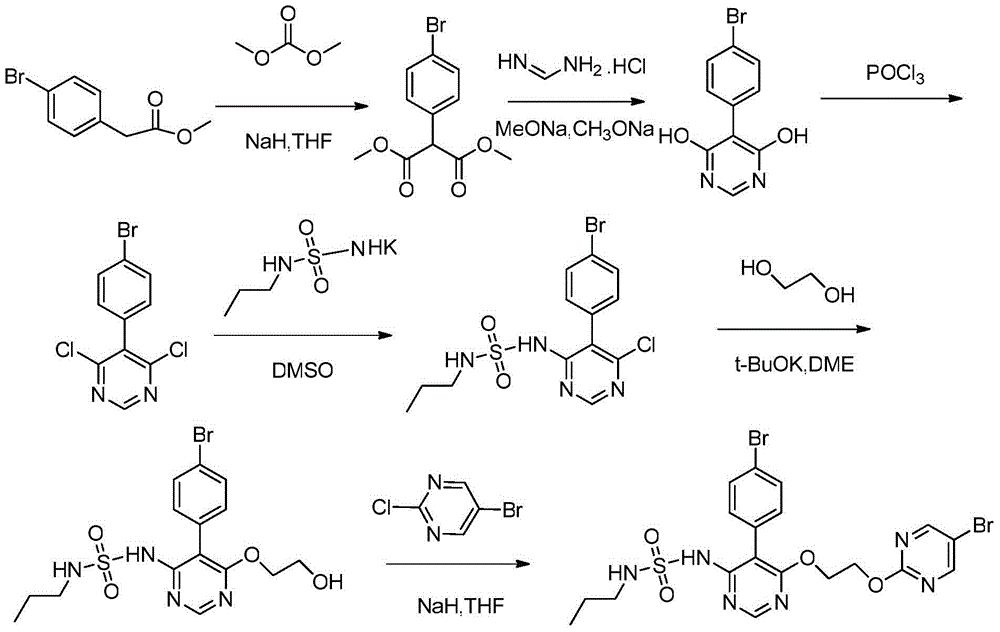
[0038] a) Preparation of compound 3: 2-[(5-(4-bromophenyl)-6-chloro-4-pyrimidinyl)oxy]ethanol
[0039] Add 10mL of N,N-dimethylformamide and 1.0g of sodium hydrogen (60%) into a 50mL three-necked flask, cool to 0°C with icy brine under stirring, add 0.62g of ethylene glycol (compound 2) dropwise, and stir for 10min after the addition ; Add dropwise a solution of 15mL N,N-dimethylformamide and 3g 5-(4-bromophenyl)-4,6-dichloropyrimidine (compound 1) at 0°C under temperature control, and stir for 5h ; Slowly add 30mL saturated ammonium chloride solution, after the addition, stir for 10min; extract with 3×30mL ethyl acetate, combine the organic layer, wash with 3×30mL water; dry the organic layer with 5g anhydrous magnesium sulfate; , the residue was passed through the column with ethyl acetate and petroleum ether to obtain 2.5 g of compound 3: 2-[(5-(4-bromophenyl)-6-chloro-4-pyrimidinyl)oxy]ethanol;
[0040] [M+H] + = 329;
[0041] 1 HNMR (CDCl 3 ): δ3.80~3.84(...
Embodiment 2
[0051]
[0052] A) Preparation of compound 8: 2-[(5-bromo-2-pyrimidinyl)oxy]ethanol
[0053] Add 50mL tetrahydrofuran, 2.1g 5-bromo-2-chloropyrimidine (compound 6), 4.3g benzotriazole-1-tris(trimethylamino)-trifluorophosphate and 3.3g cesium carbonate into a 100mL three-necked flask, and stir for 1h ; Add 11g ethylene glycol (compound 2) and 3.3g cesium carbonate, stir the reaction at room temperature for 10h; slowly add 100mL water, extract with 3×50mL ethyl acetate, combine the organic layers, wash with 3×30mL water; 5g anhydrous Dry the organic layer with magnesium sulfate; rotary evaporate in a water bath at 40°C, and pass the residue through a column with ethyl acetate and petroleum ether to obtain 1.4 g of oil compound 8: 2-[(5-bromo-2-pyrimidinyl)oxy] ethanol;
[0054] [M+H] + = 219;
[0055] 1 HNMR (CDCl 3 ): δ3.90~3.96 (2H, m), δ4.43~4.47 (2H, m), δ8.52 (2H, s).
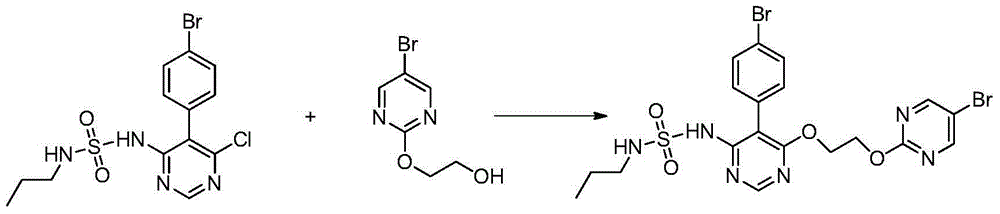
[0056] B) Preparation of compound 10: 5-(4-bromophenyl)-4-[2-[(5-bromo-2-pyrimidinyl)oxy]ethanol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com