Macitentan crystal and its preparation method, its pharmaceutical composition and use
A technology of macitentan and its composition, which is applied in the field of pharmaceutical chemical crystallization, can solve the problems of affecting drug efficacy, poor solubility, and slow dissolution rate of tablets, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0125] N-[5-(4-bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]- Form I of ethoxy]-4-pyrimidinyl]-N'-propylsulfonamide (macitentan). Specifically:
[0126] Sodium hydride (1.67g, 69.6mmol, 55% content in mineral oil) was washed twice with 10mL of n-hexane, the n-hexane solution was discarded, and the washed sodium hydride was dissolved in 200mL of tetrahydrofuran, and N-[5-(4 -Bromophenyl)-6-[2-[(hydroxyethoxy)-4-pyrimidinyl]-N'-propylsulfonamide (10.0g, 23.2mmol), the mixture was stirred for 15 minutes, diluted with 400mL DMF, and finally 5-Bromo-2-chloropyrimidine (5.38 g, 27.8 mmol) was added, the temperature of the reaction solution was raised to 60° C., and the temperature was maintained for 2 hours, and the reaction was completed. Pour the reaction solution into 250 mL of 10% citric acid aqueous solution, add ethyl acetate to extract twice, each time 300 mL of ethyl acetate, combine the organic phases, wash with water twice, each time 200 mL of water, add magnesium sulfa...
Embodiment 1
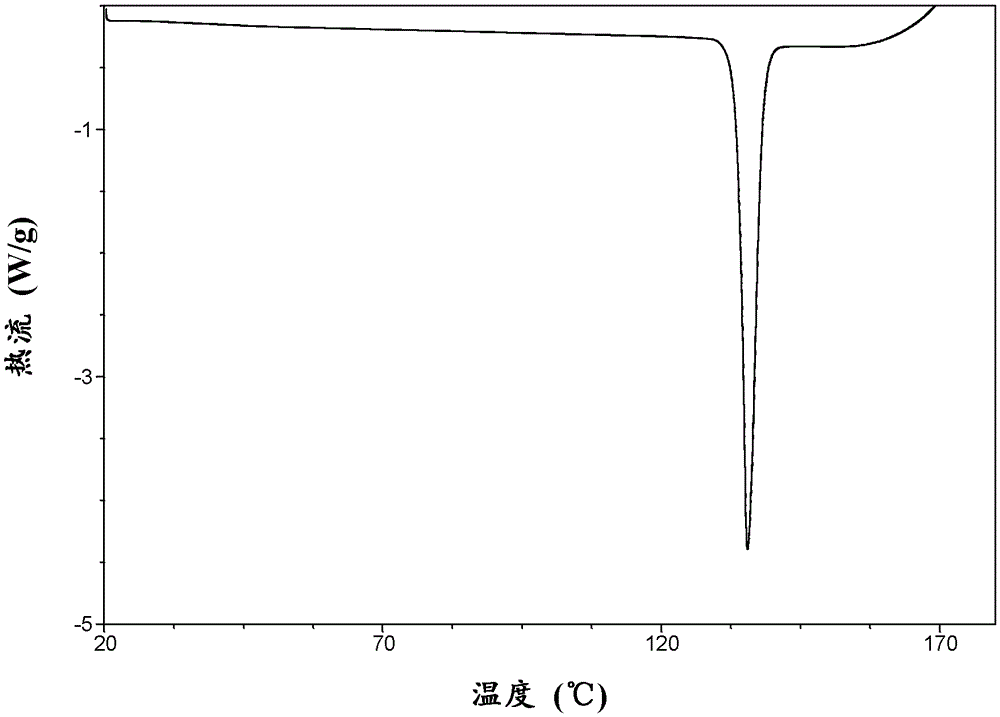
[0131] Take 9.8mg of macitentan in a 5mL glass vial, add 0.9mL of methanol to form a suspension, stir at 60°C for 1 day, white crystals precipitate, separate by filtration, wash twice with methanol, and dry in vacuum at room temperature for 1 hour. Crystals of macitentan methanolate were obtained. Yield 8.6 mg, 85% yield.
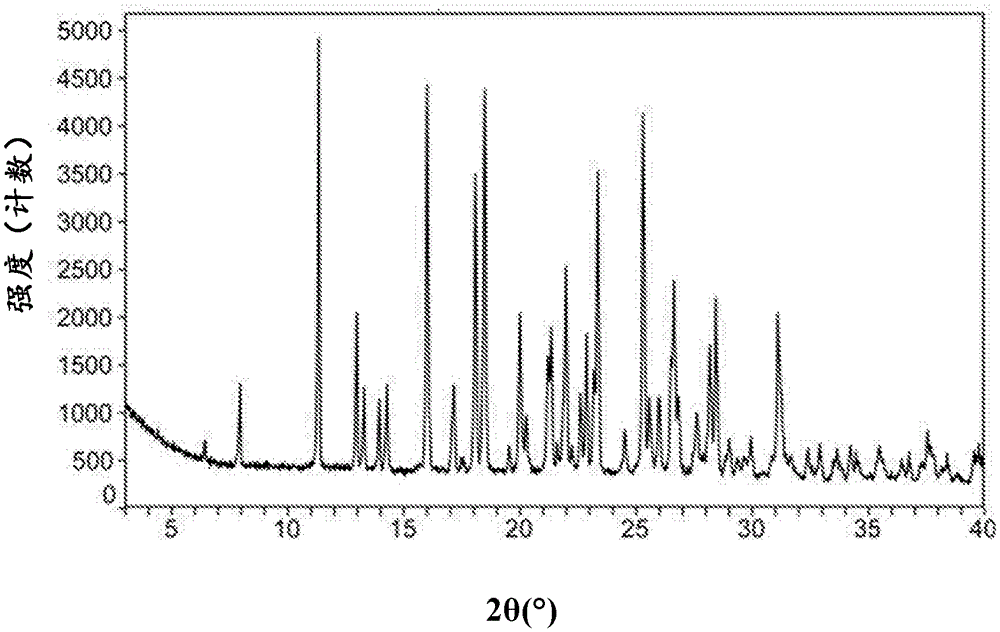
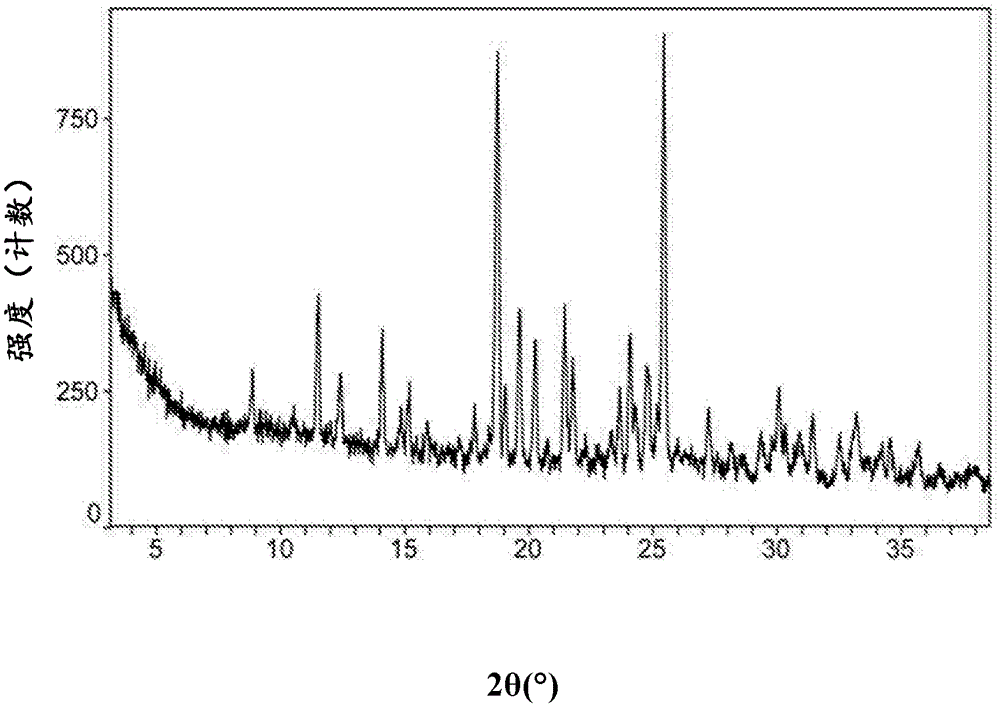
[0132] XRPD patterns such as Figure 8 shown.
[0133] 1 HNMR (CDCl 3 ): 8.52(s, 2H), 8.50(s, 1H), 7.59-7.61(m, 2H), 7.17-7.19(m, 2H), 5.65(t, J=6.2Hz, 1H), 4.73-4.76( m, 2H), 4.63-4.65(m, 2H), 3.52(s, 2H), 2.98(q, J=6.8Hz, 2H), 1.58-1.63(m, 2H), 1.30-1.52(m, 7H) , 0.97 (t, J=7.4Hz, 3H). It shows that compared with the macitentan crystal form I prepared in Preparation Example 1, the macitentan methanolate crystal contains methanol, and each molecule of macitentan contains about 2 / 3 methanol molecules.
[0134] PLM map such as Figure 9 As shown, it has a better morphology.
Embodiment 2
[0136] Take 50.0 mg of macitentan in a 5 mL glass vial, add 0.5 mL of methanol to form a suspension, stir at room temperature for 7 days, precipitate white crystals, separate by filtration, wash with methanol for 3 times, and dry in vacuum at room temperature for 24 hours to obtain macitentan Tetan methanolate crystals, yield 48.1mg, yield 93%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com