Macitentan microsphere and preparation method thereof
A technology of macitentan and microspheres, applied in the field of macitentan microsphere preparation and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
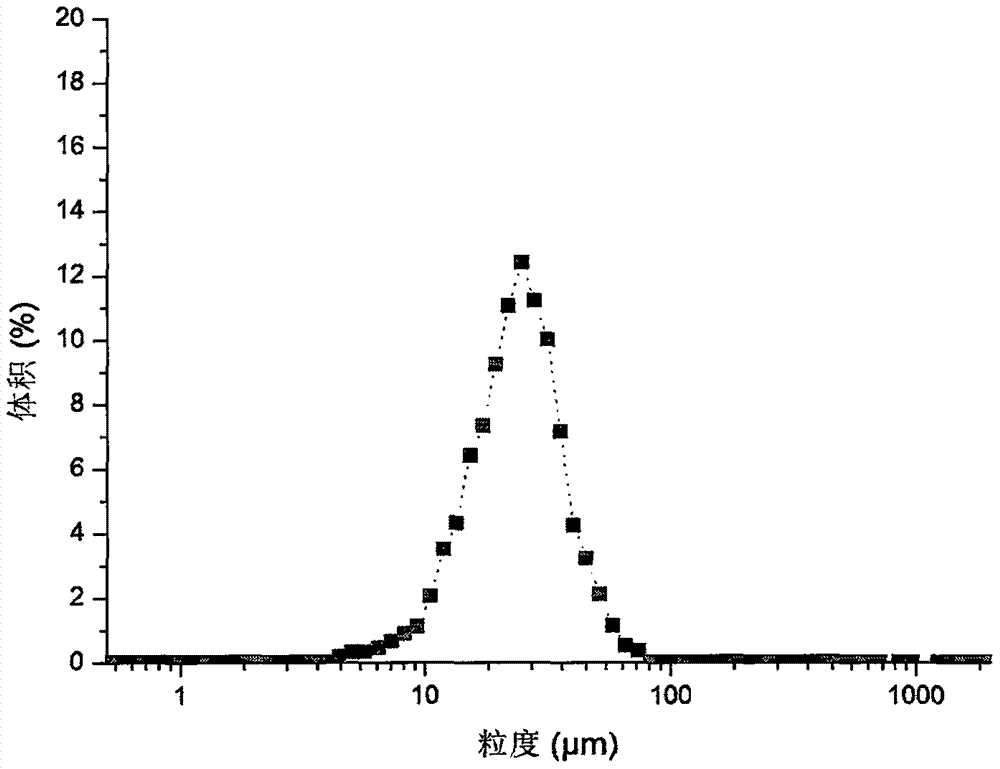
[0044] The drug-loaded microspheres were prepared by emulsification-solvent evaporation method. Weigh 600mg PLGA and 30mg macitentan and dissolve it in 2.82g dichloromethane, as the oil phase, take 0.5% PVA aqueous solution as the water phase, add the oil phase to the water phase at a ratio of 1:15 at room temperature, High shear at 7000rpm for 120s to form an O / W emulsion. The obtained O / W emulsion was added to the 0.1% PVP aqueous solution in a stirring state at a volume ratio of 1:3, and the stirring was continued for 3 h to fully volatilize the dichloromethane to obtain the macitentan microspheres of the present invention. Subsequently, the macitentan microspheres were centrifuged, washed three times with distilled water, and dried.
[0045] The encapsulation efficiency, drug loading and particle size of the obtained microspheres were determined by the following methods.
[0046] Weigh 5 mg of microspheres into a 15 mL centrifuge tube, add 0.5 mL of dichloromethane, soni...
Embodiment 2
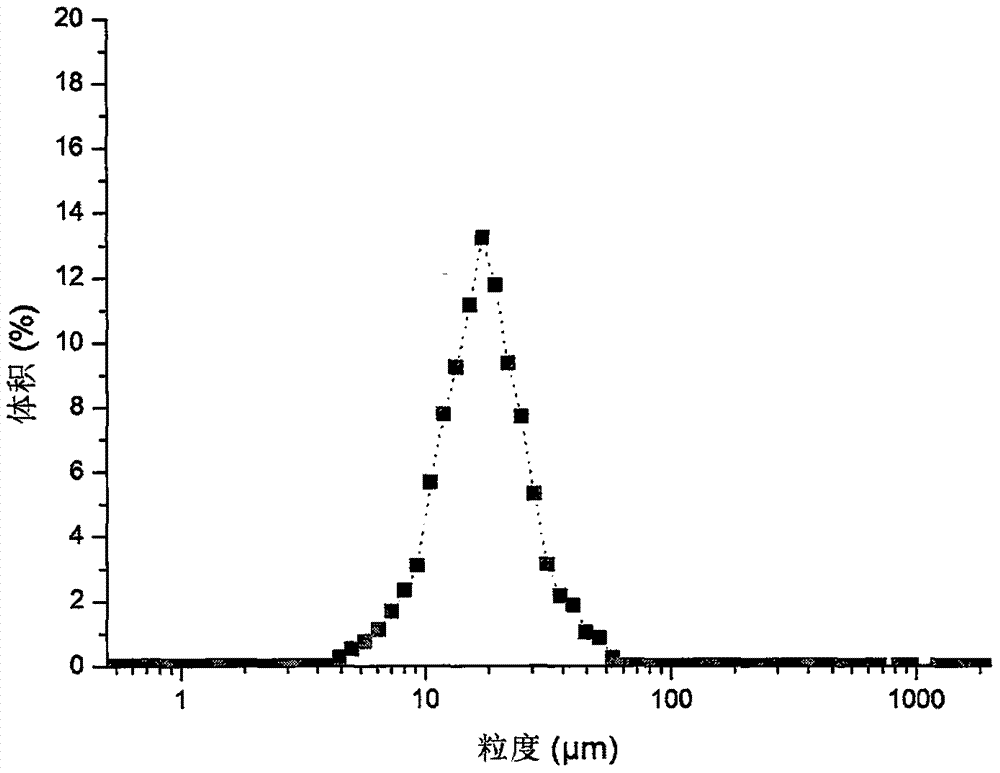
[0052] Drug-loaded microspheres were prepared in the same manner as in Example 1, except that the shear rate was 8500 rpm. It was determined that the encapsulation efficiency of the obtained microspheres was 93.76%, the drug loading was 4.37%, the average particle size was 16.8 μm, and 91.6% of the microspheres had a particle size distribution of 7-30 μm. figure 2 .
Embodiment 3
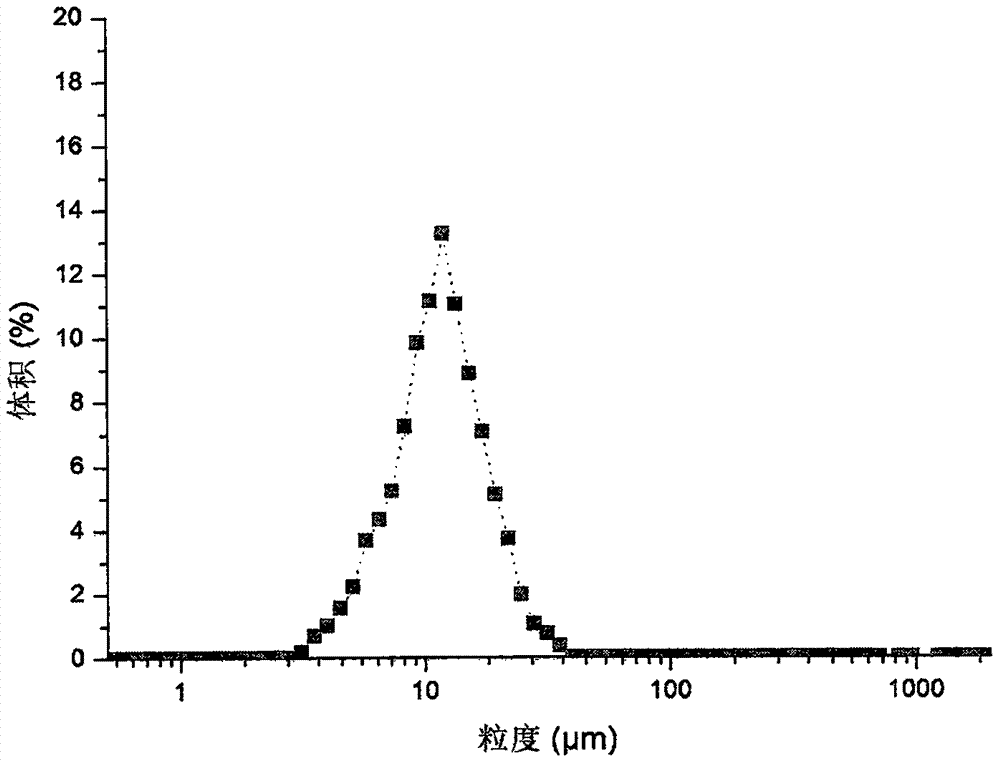
[0054]Drug-loaded microspheres were prepared in the same manner as in Example 1, except that the shear rate was 10,000 rpm. It was determined that the encapsulation efficiency of the obtained microspheres was 85.76%, the drug loading was 4.15%, the average particle size was 11.6 μm, and 86.3% of the microspheres had a particle size distribution of 7-30 μm. image 3 .
[0055] It can be seen from the results of Examples 1-3 that the size of the shear rate affects the particle size of the microspheres: in the range of 7000rpm-10000rpm, the larger the shear rate, the smaller the particle size of the microspheres. Most preferably, the shear rate is 8500 rpm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com