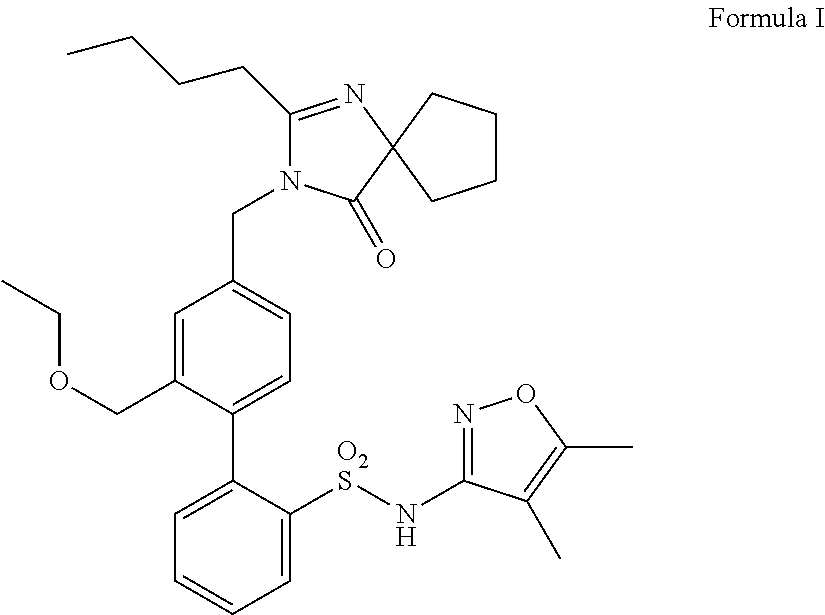
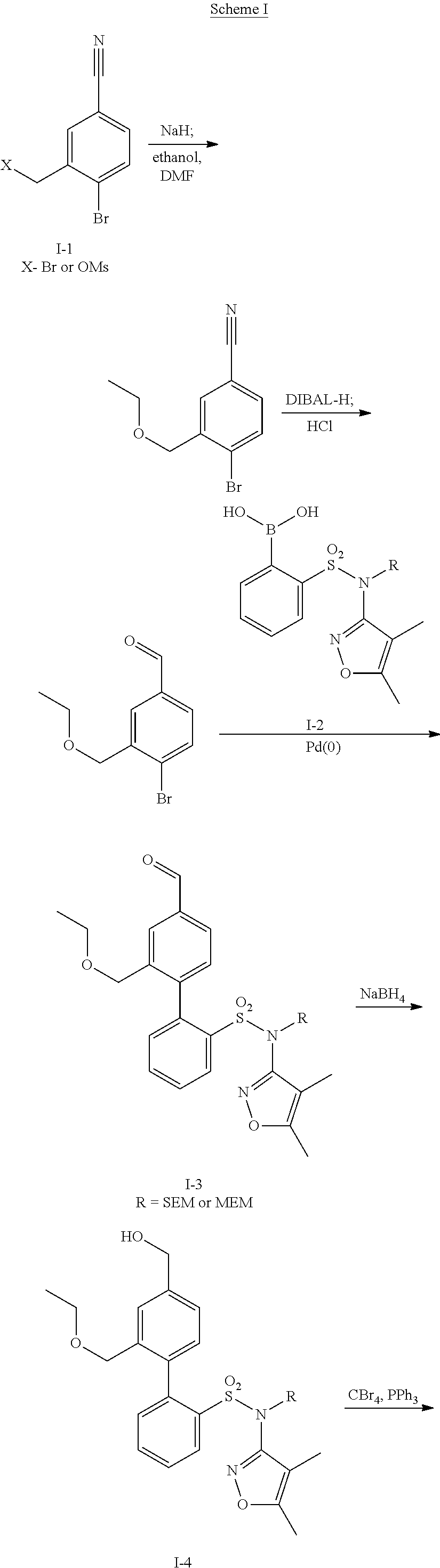
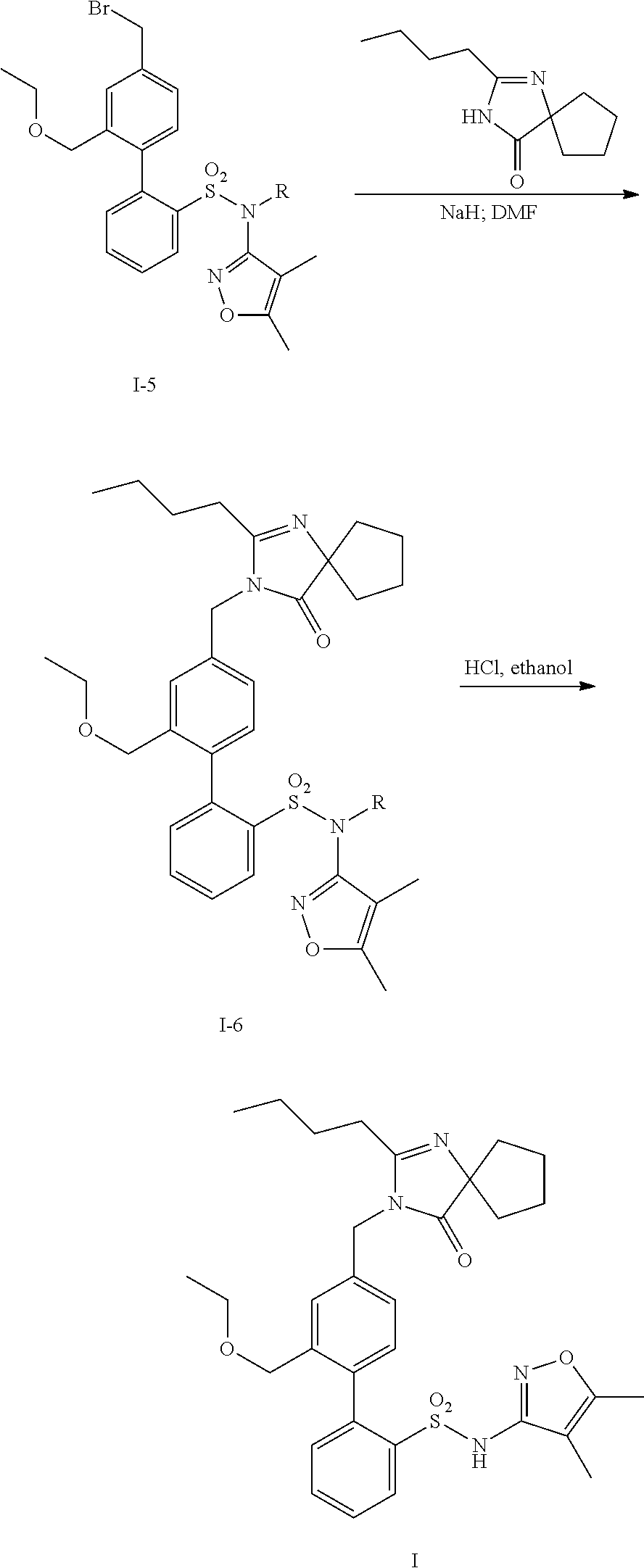
Oral formulations of diphenylsulfonamide endothelin and angiotensin ii receptor agonists to treat elevated blood pressure and diabetic nephropathy
a diphenylsulfonamide and endothelin technology, applied in the field of biphenyl sulfonamide compounds, can solve the problems of limiting the production of et-1, and achieve the effects of improving friability, dissolvability, and uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0209]
Name: 4′-[(2-Butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl]-2′-formyl-N-(4,5-dimethyl-3-isoxazolyl)-[[1,1′-biphenyl]-2-sulfonamide]
A. 4′-[(2-Butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl]-2′-formyl-N-(4,5-dimethyl-3-isoxazolyl)-N-(2-methoxyethoxymethyl)[1,1′-biphenyl]-2-sulfonamide
[0210]Palladium catalyzed Suzuki coupling of 4-bromo-3-(ethoxymethyl)benzaldehyde and [2-[[(4,5-dimethyl-3-isoxazolyl)[(2-methoxyethoxy)methyl]amino]sulfonyl]phenyl]boronic acid was performed according to General Method 3 to afford N-(4,5-dimethyl-3-isoxazolyl)-4′-(ethoxycarbonyl)-2′-(formyl)-N-((methoxyethoxy)methyl)[1,1′-biphenyl]-2-sulfonamide (81%) following silica-gel chromatography.
B. 4′-[(2-Butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl]-2′-formyl-N-(4,5-dimethyl-3-isoxazolyl)-[1,1′-biphenyl]-2-sulfonamide
[0211]Treatment of N-(4,5-dimethyl-3-isoxazolyl)-4′-(ethoxycarbonyl)-2′-(formyl)-N-((methoxyethoxy)methyl)[1,1′-biphenyl]-2-sulfonamide with 6N aqueous hydrochloric acid acco...
example 2
[0212]4′-[(2-Butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl]-N-(4,5-dimethyl-3-isoxazolyl)-2′-(ethoxymethyl)-[1,1′-biphenyl]-2-sulfonamide was synthesized by combinations of the General Methods.
Name: 4′-[(2-Butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl]-N-(4,5-dimethyl-3-isoxazolyl)-2′-(ethoxymethyl)-[1,1′-biphenyl]-2-sulfonamide
[0213]
[0214]Starting Material: X—Br or OMs
[0215]General Methods Applied (yield, %): General Method 1, EtOH (77); General Method 2 (80); General Method 3 (70); General Method 4 (98); General Method 5 (80); General Method 1 (83); General Method 6 (86)
[0216]M / z (MH)+: 593
[0217]HPLC % Purity: >98
[0218]HPLC retention time, min (HPLC method): 18.75 (E)
example 3
4′-[(2-Butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl]-N-(4,5-dimethyl-3-isoxazolyl)-2′-(ethoxymethyl)[1,1′-biphenyl]-2-sulfonamide [crystalline]
[0219]
Alternative Synthesis of 4′-[(2-Butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl]-N-(4,5-dimethyl-3-isoxazolyl)-2′-(ethoxymethyl)[1,1′-biphenyl]-2-sulfonamide
Step A. Ethyl 4-bromo-3-(bromomethyl)benzoate
[0220]Ethyl 4-bromo-3-methylbenzoate (110 g, 450 mmol.) was treated with NBS according to the procedure of Example 5. Silica gel chromatography with hexanes / ethyl acetate as eluant provided ethyl 4-bromo-3-(bromomethyl)benzoate (91 g, 62%) as a white solid.
Step B. Ethyl 4-bromo-3-(ethoxymethyl)benzoate
[0221]A solution of ethyl 4-bromo-3-(bromomethyl)benzoate (89 g, 280 mmol.) in a mixture of ethanol (300 mL) and DMF (50 mL) was treated at 0° C. with sodium ethoxide (135 mL of a 21% solution in ethanol). The mixture was allowed to warm to rt and was stirred for 16 h. The ethanol was evaporated under reduced pressure. Ethyl aceta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com