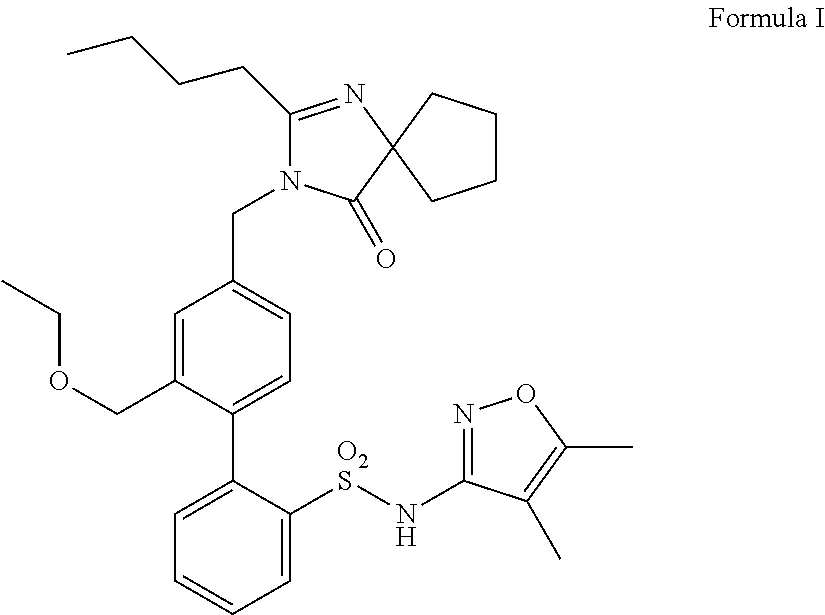
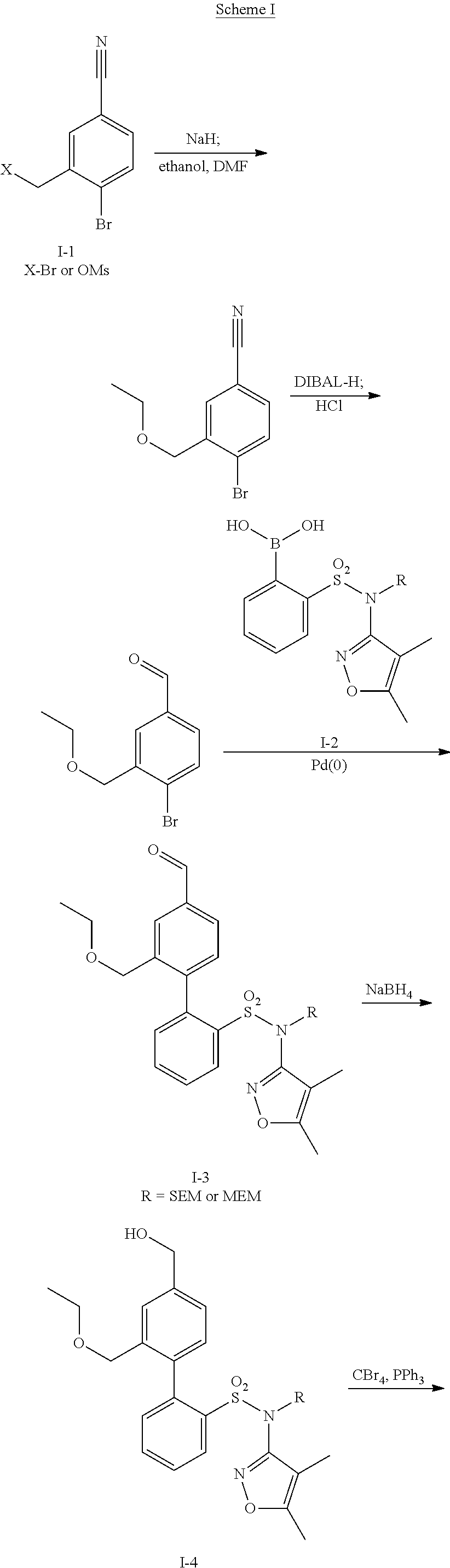
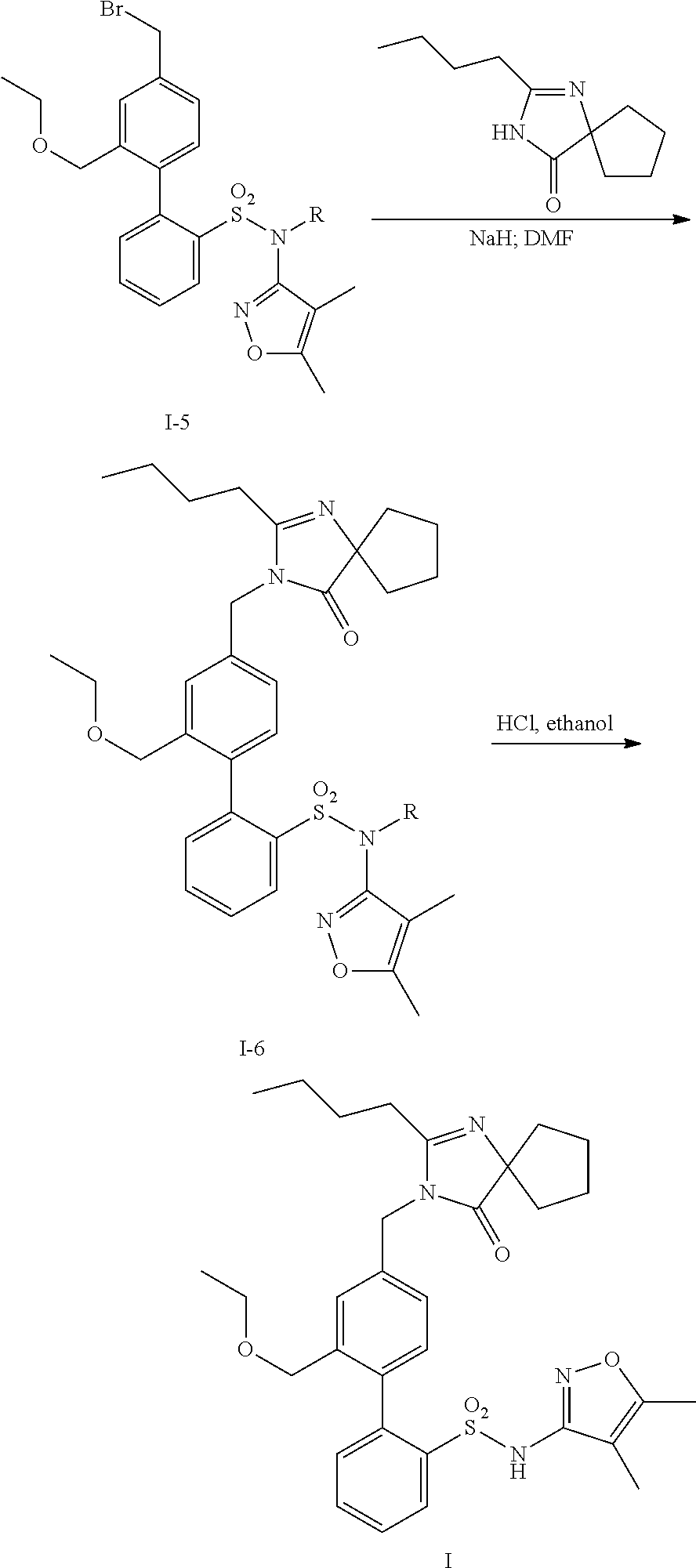
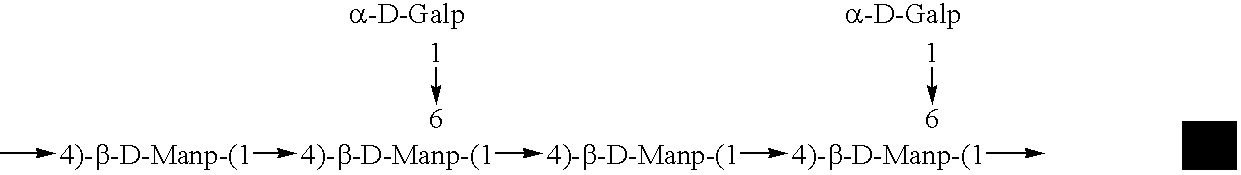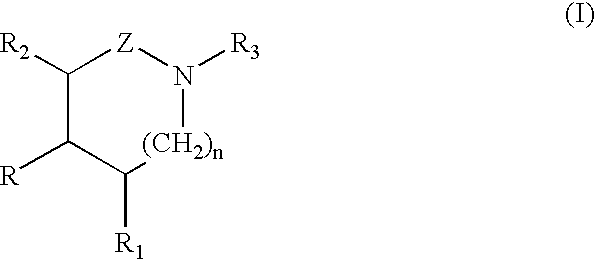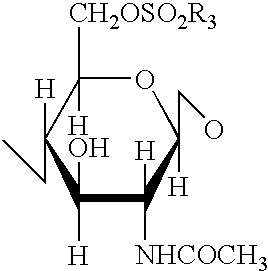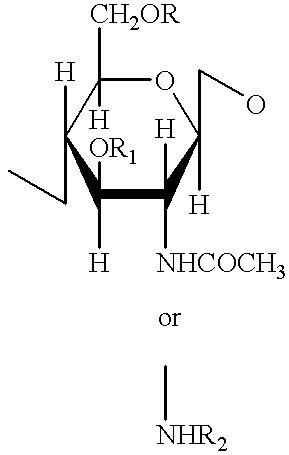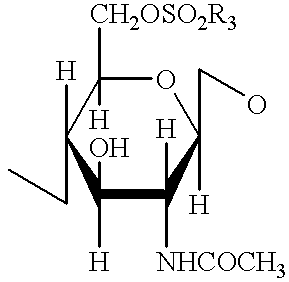Patents
Literature
215 results about "Endothelins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Endothelins are peptides with receptors and effects in many body organs. Endothelin constricts blood vessels and raises blood pressure. The endothelins are normally kept in balance by other mechanisms, but when overexpressed, they contribute to high blood pressure (hypertension), heart disease, and potentially other diseases.
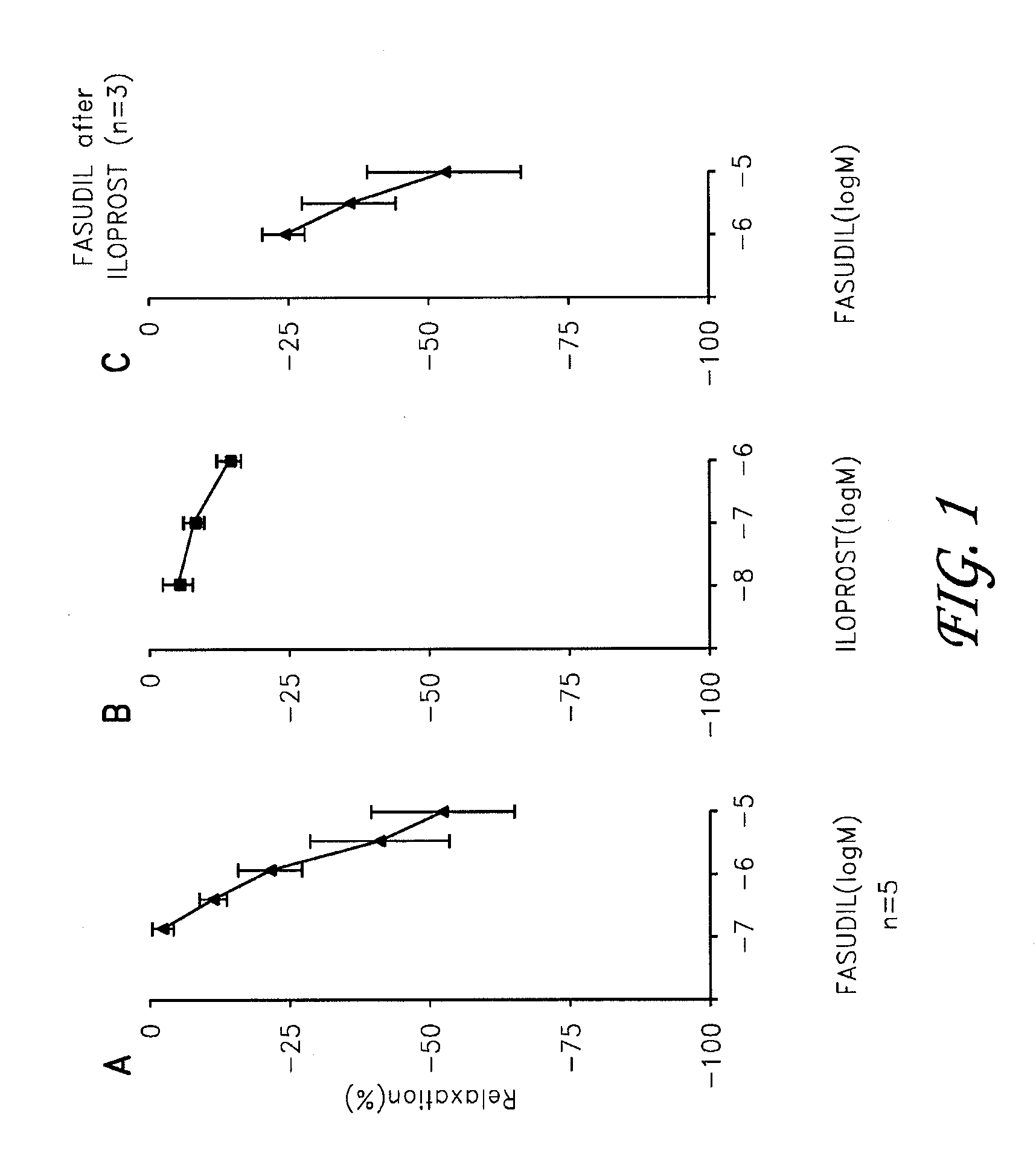
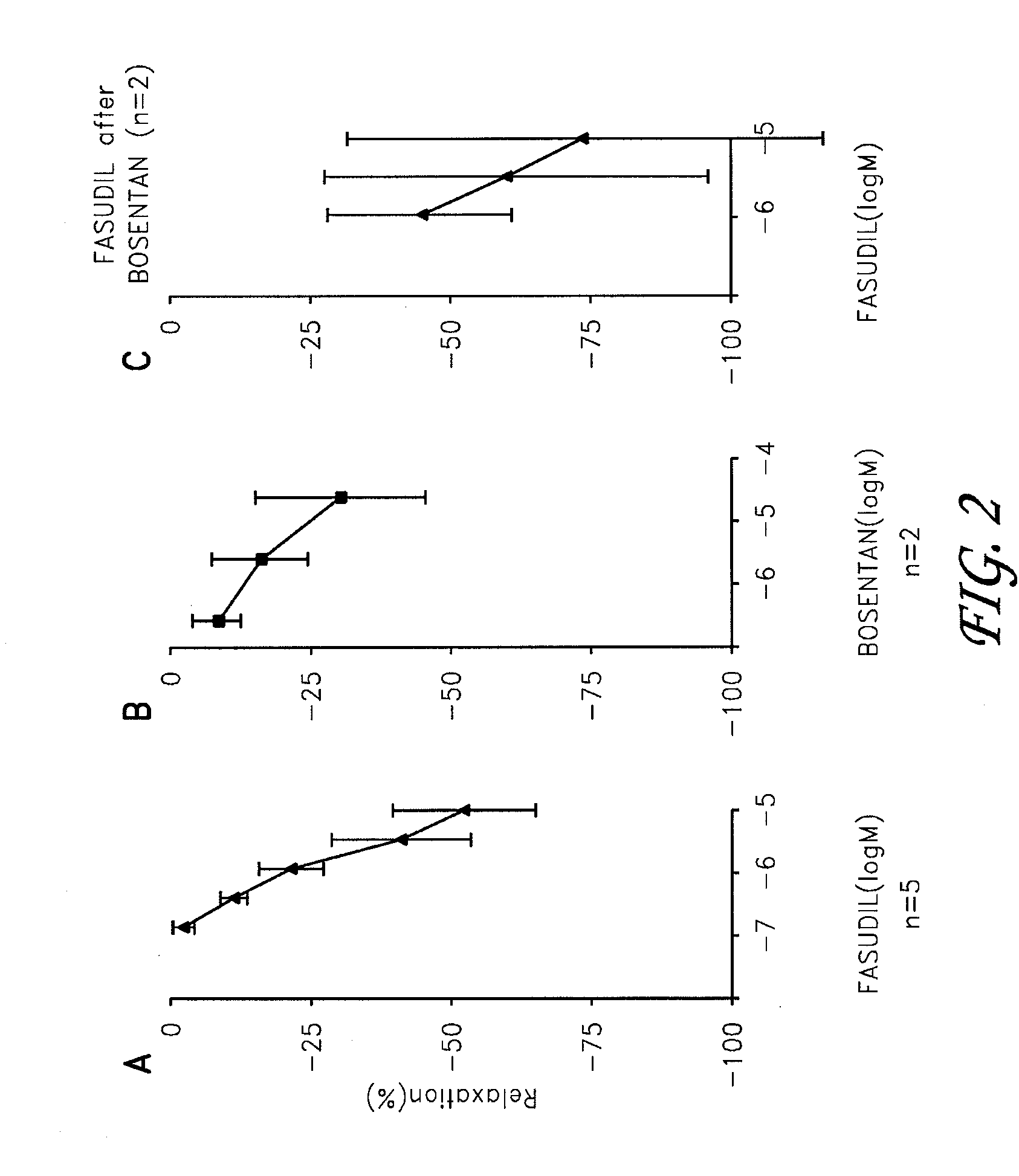
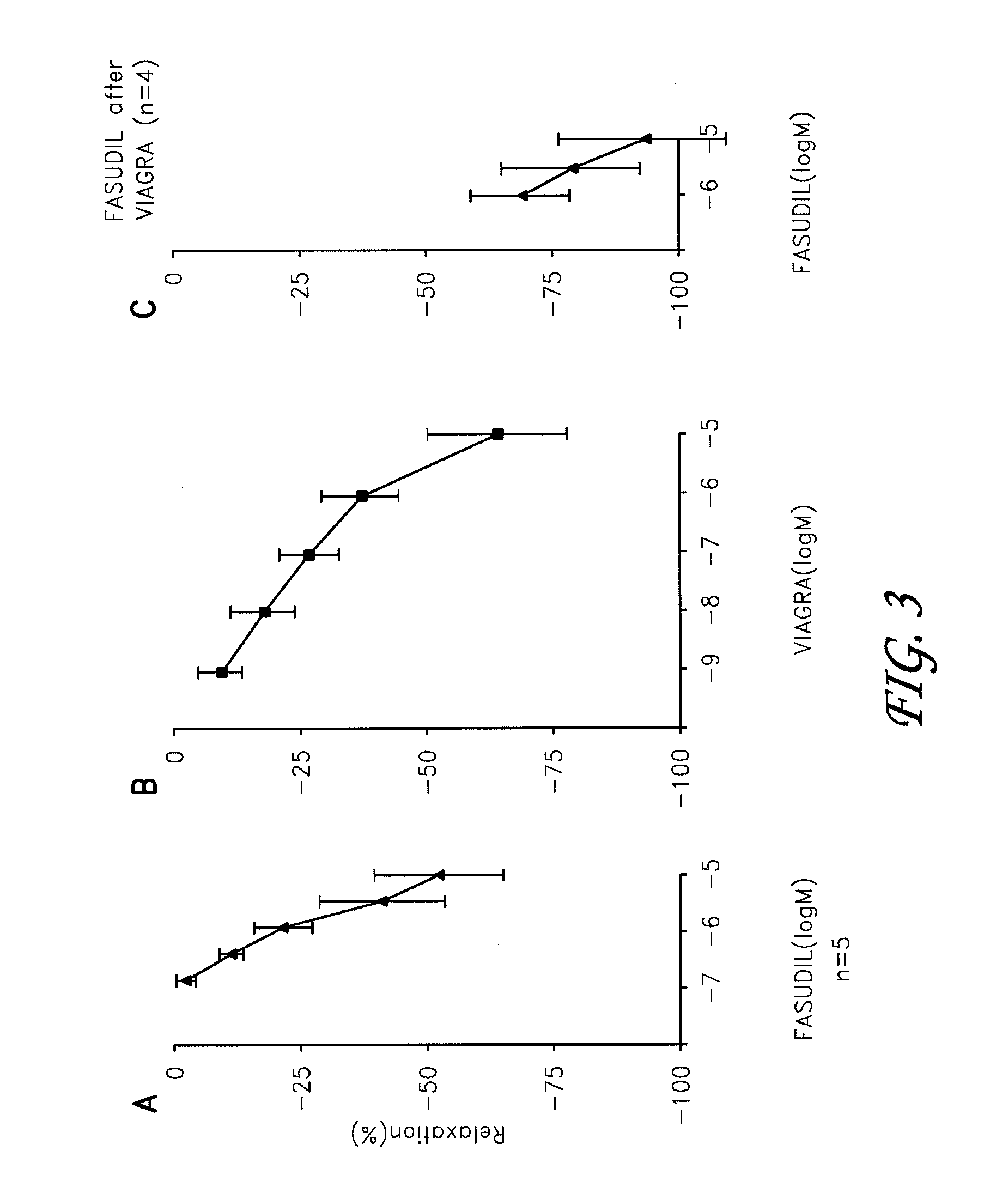
Fasudil in combination therapies for the treatment of pulmonary arterial hypertension
Preferred embodiments of the present invention are related to novel therapeutic drug combinations and methods for treating and / or preventing pulmonary arterial hypertension and / or stable angina. More particularly, aspects of the present invention are related to therapeutic combinations comprising a Rho-kinase inhibitor, such as fasudil, and one or more additional compounds selected from the group consisting of prostacyclins, such as iloprost, endothelin receptor antagonists, PDE inhibitors, calcium channel blockers, 5-HT2A antagonists, such as sarpogrelate, selective serotonin reuptake inhibitors, such as fluoxetine, statins, and vascular remodeling modulators, such as Gleevec.
Owner:ASAHI KASEI PHARMA
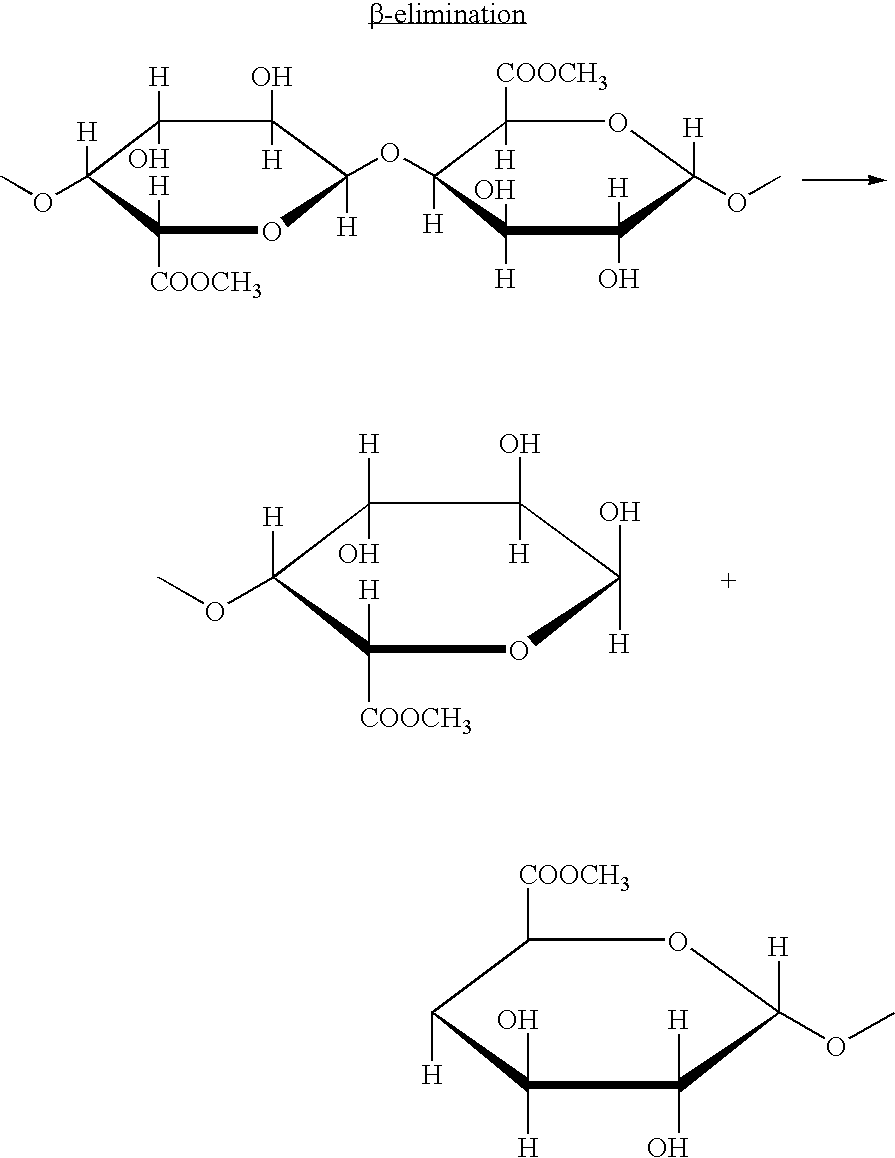
Galactose-pronged polysaccharides in a formulation for antifibrotic therapies
Methods and compositions for reducing fibrosis and cirrhosis are provided in which an effective dose of an admixture of a polysaccharide compound and, for example, a compound selected from the group consisting of antibodies specific to intracellular or cell-surface: (i) beta-PDGF receptors; (ii) synaptophysin; (iii) zvegf3; (iv) CCR1 receptors; (v) connective tissue growth factor; (vi) alpha 1-smooth muscle actin; (vii) matrix metalloproteinases MMP 2 and MMP9; (viii) matrix metalloproteinase inhibitors TIMP1 and TMP2; (ix) integrins; (x) TFG-β1; (xi) endothelin receptor antagonists; and (xii) collagen synthesis and degradation modulating compounds; (xiii) actin synthesis and degradation modulating compounds; and (xiv) tyrosine kinases is administered to an animal in order to treat fibrosis.
Owner:GALECTIN THERAPEUTICS
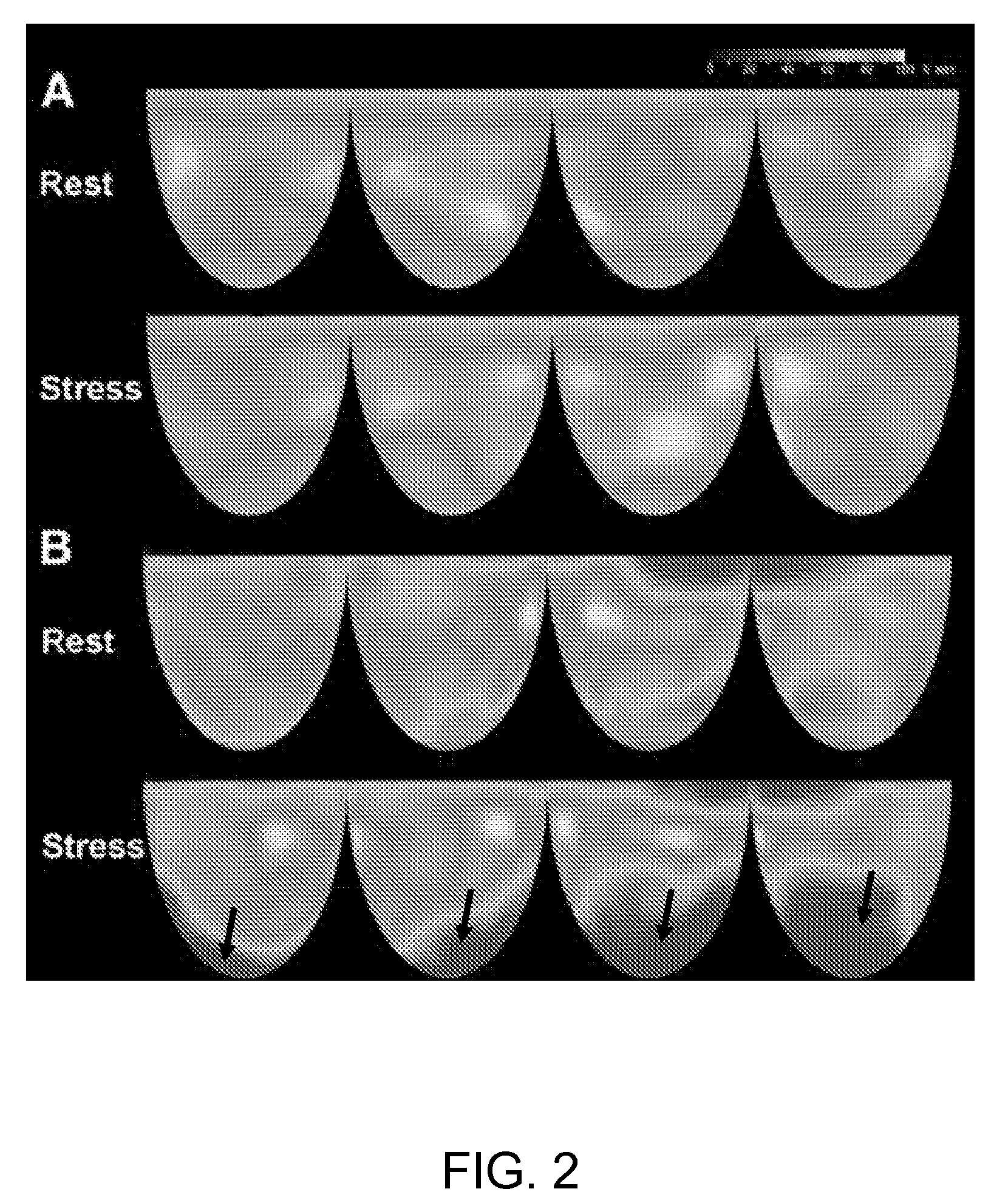
Method for detecting coronary endothelial dysfunction and early atherosclerosis
InactiveUS20080058642A1Reduce incidenceReduce riskTomographyAngiographyTherapeutic treatmentCoronary Artery Disease Risk
A method of detecting endothelin receptor A mediated coronary microvascular endothelial dysfunction in an asymptomatic subject is disclosed. The method comprises obtaining sets of noninvasive cardiac PET perfusion images of the subject before and after administration of selective endothelin receptor A (ETA receptor) antagonist. The images are analyzed, including application of applying Markovian homogeneity analysis, and the results are compared to detect improvement, or lack of improvement, of myocardial perfusion homogeneity in the subject. A result of improved myocardial perfusion homogeneity after administration of the antagonist indicates the presence of ETA receptor-mediated microvascular endothelial dysfunction in the subject and indicates therapeutic treatment to improve endothelial function and / or to reduce coronary artery disease risk factors.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Endothelin antagonists
Owner:ABBVIE INC
Endothelin antagonists
InactiveUS7208517B1Suppression problemAvoid problemsBiocideAnimal repellantsLymphatic SpreadMedicine
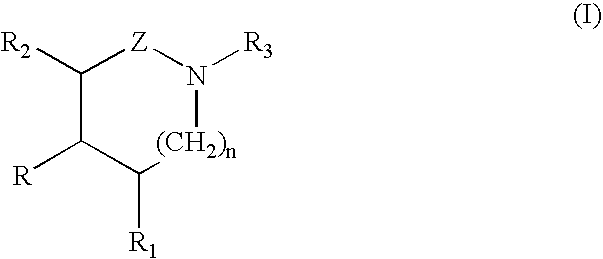
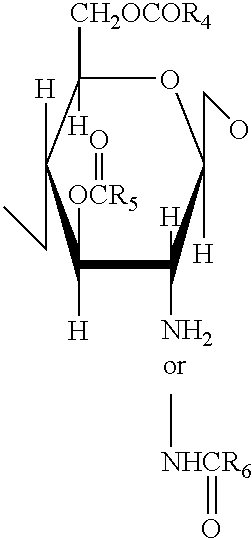
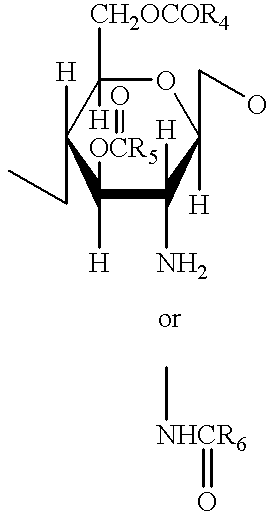
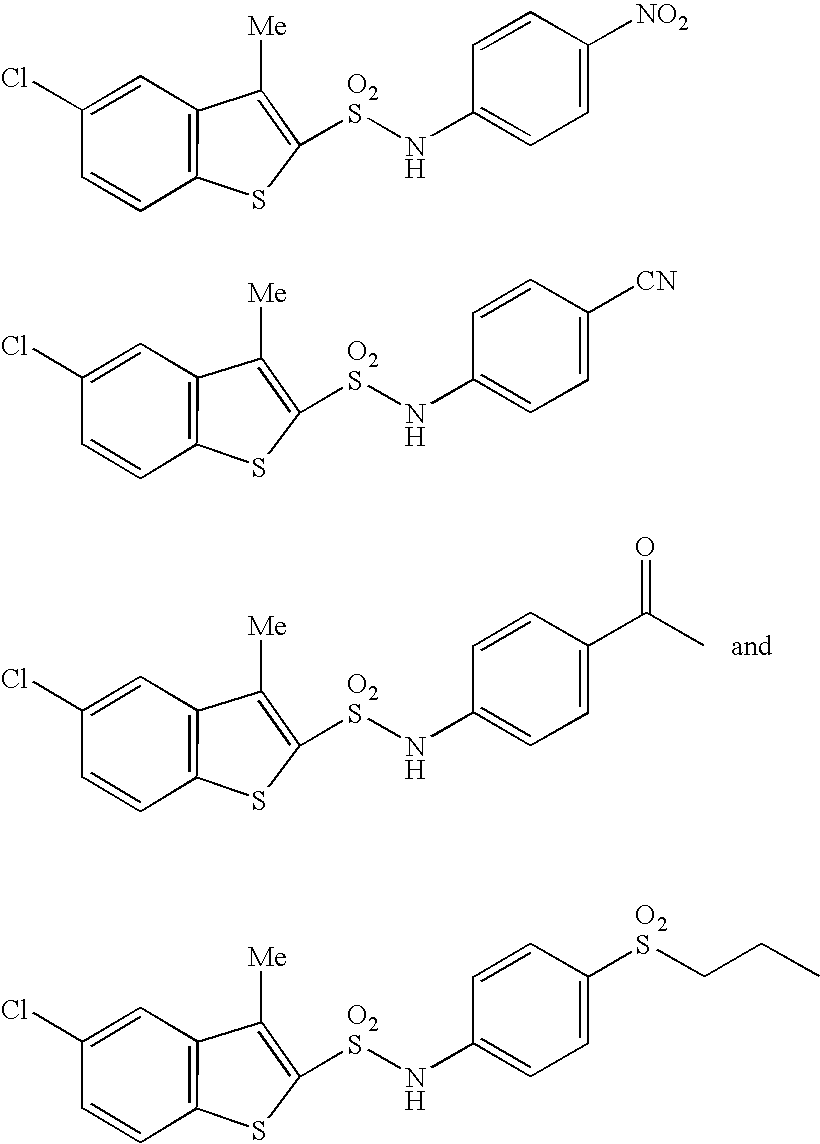
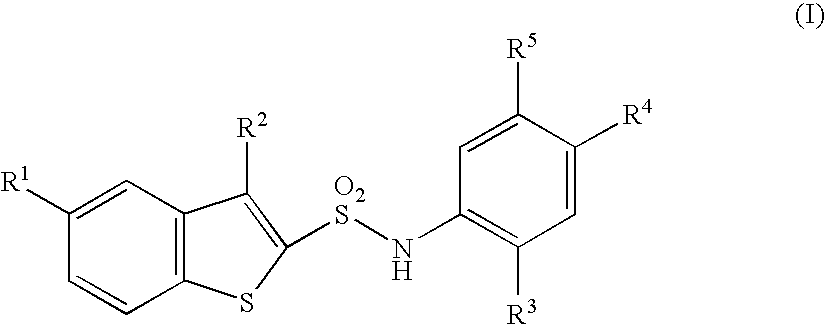
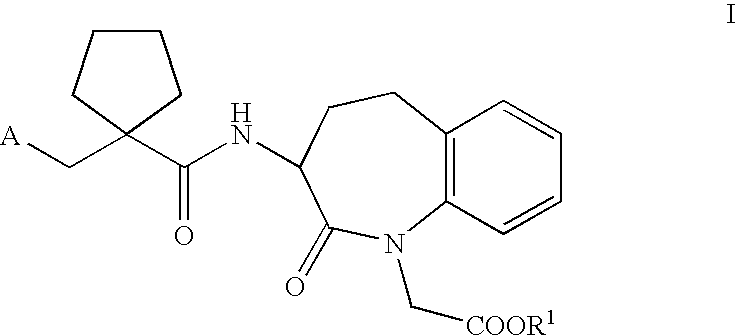
A compound of the formula (I):or a pharmaceutically acceptable salt thereof is disclosed, as well as processes for and intermediates in the preparation thereof, a method of antagonizing endothelin, methods for the inhibition of bone metastases, methods for the prevention of growth of new metastases, methods for the inhibition of bone turnover, and methods for the prevention of bone loss in patients, including cancer patients, using an endothelin ET-A receptor antagonist.
Owner:ABBVIE INC
Methods for treating a breach or puncture in a blood vessel
InactiveUS20030078234A1CessationIncreased riskBiocideSolution deliveryArteriolar VasoconstrictionVascular structure
The present invention relates to compositions comprising semi-crystalline beta-1-4-N-acetylglucosamine polymers (p-GlcNac) and methods utilizing such polymers modulation of vascular structure and / or function. The compositions and methods disclosed are useful for stimulating, in a p-GlcNac concentration-dependent manner, endothelin-1 release, vasoconstriction, and / or reduction in blood flow out of a breached vessel, as well as for contributing to or effecting cessation of bleeding. The methods of the present invention comprise topical administration of materials comprising semi-crystalline p-GlcNac polymers that are free of proteins, and substantially free of single amino acids as well as other organic and inorganic contaminants, and whose constituent monosaccharide sugars are attached in a beta-1-4 conformation.
Owner:MARINE POLYMER TECH
Use of endothelin antagonists to prevent restenosis
InactiveUS20050175667A1Improve efficiencyImprove efficacyBiocideOrganic active ingredientsPercent Diameter StenosisThrombus
Provided are devices and methods for treating or preventing smooth muscle cell proliferation caused by endothelin-mediated conditions. In particular, a medical device comprising a structure which is implantable within a body lumen and means on or within the structure for releasing an endothelin (A) receptor antagonist at a rate effective to inhibit smooth muscle cell proliferation. The device can be, for example, an expansible stent or a graft, and the means can include a matrix coating, wherein the endothelin (A) receptor antagonist can be dispersed within the coating or disposed directly on the structure and under the matrix. The methods and devices of this invention can be used to decrease the incidence of restenosis as well as other thromboembolic complications resulting from implantation of medical devices.
Owner:EDWARDS LIFESCIENCES LLC
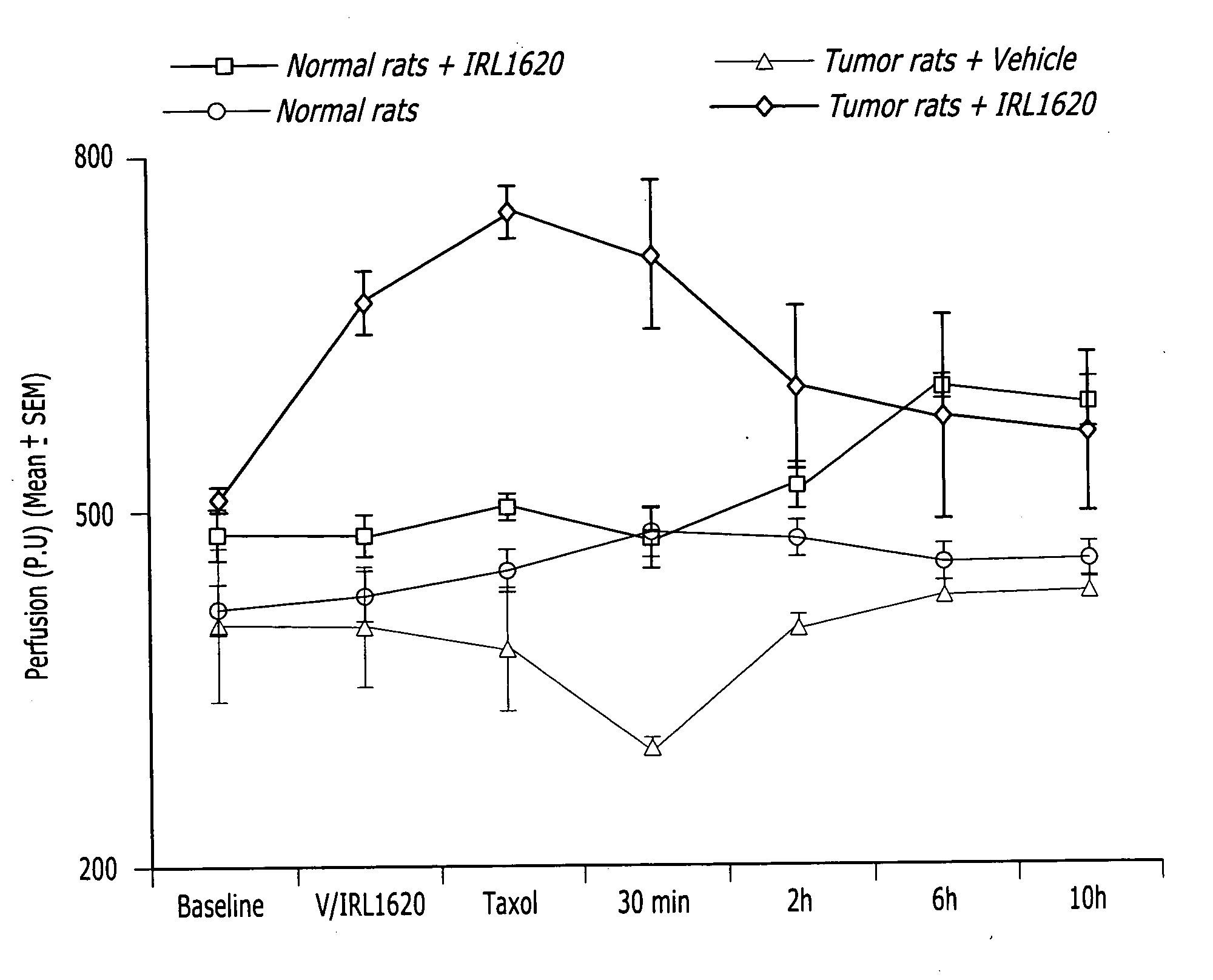
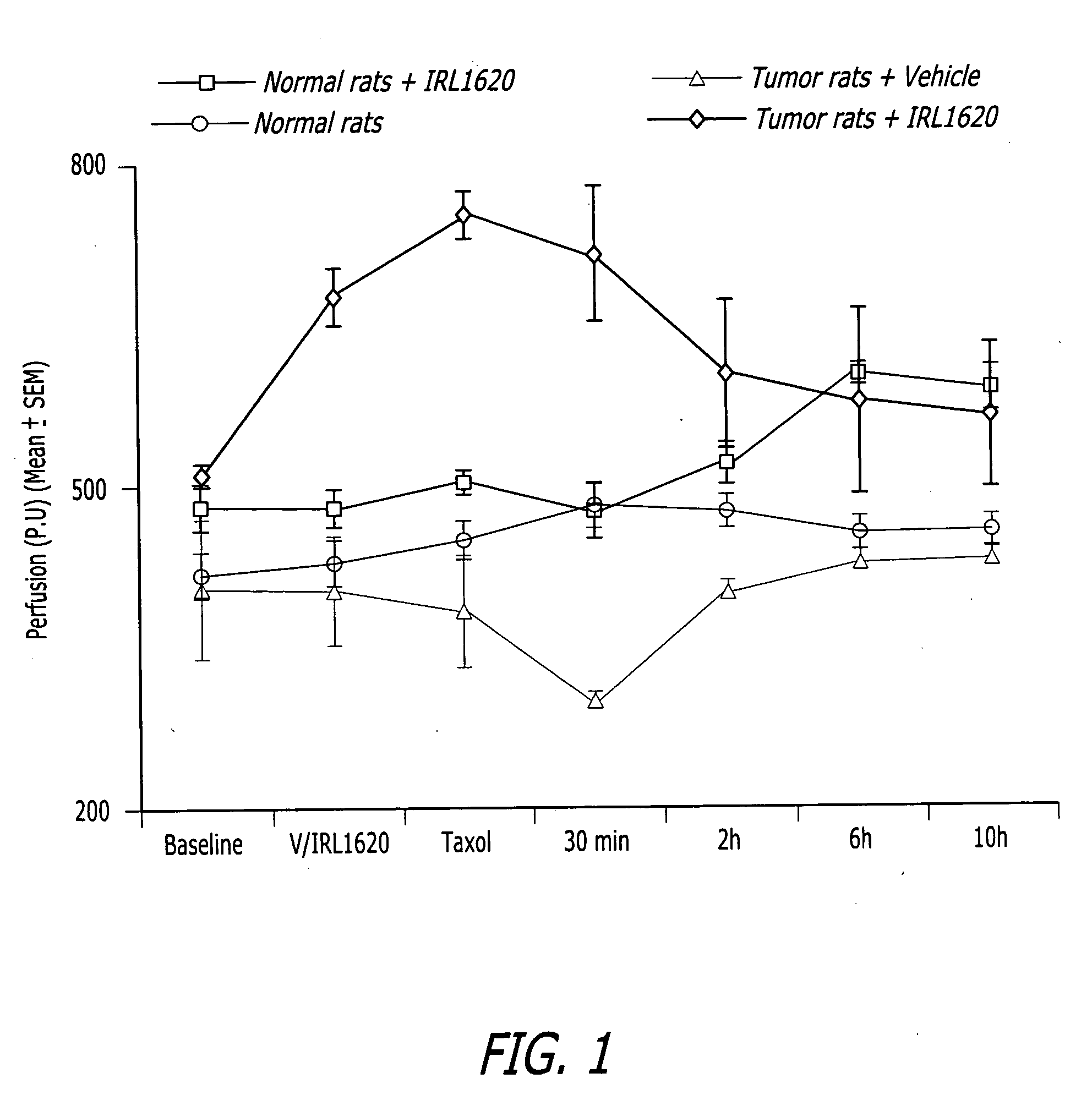
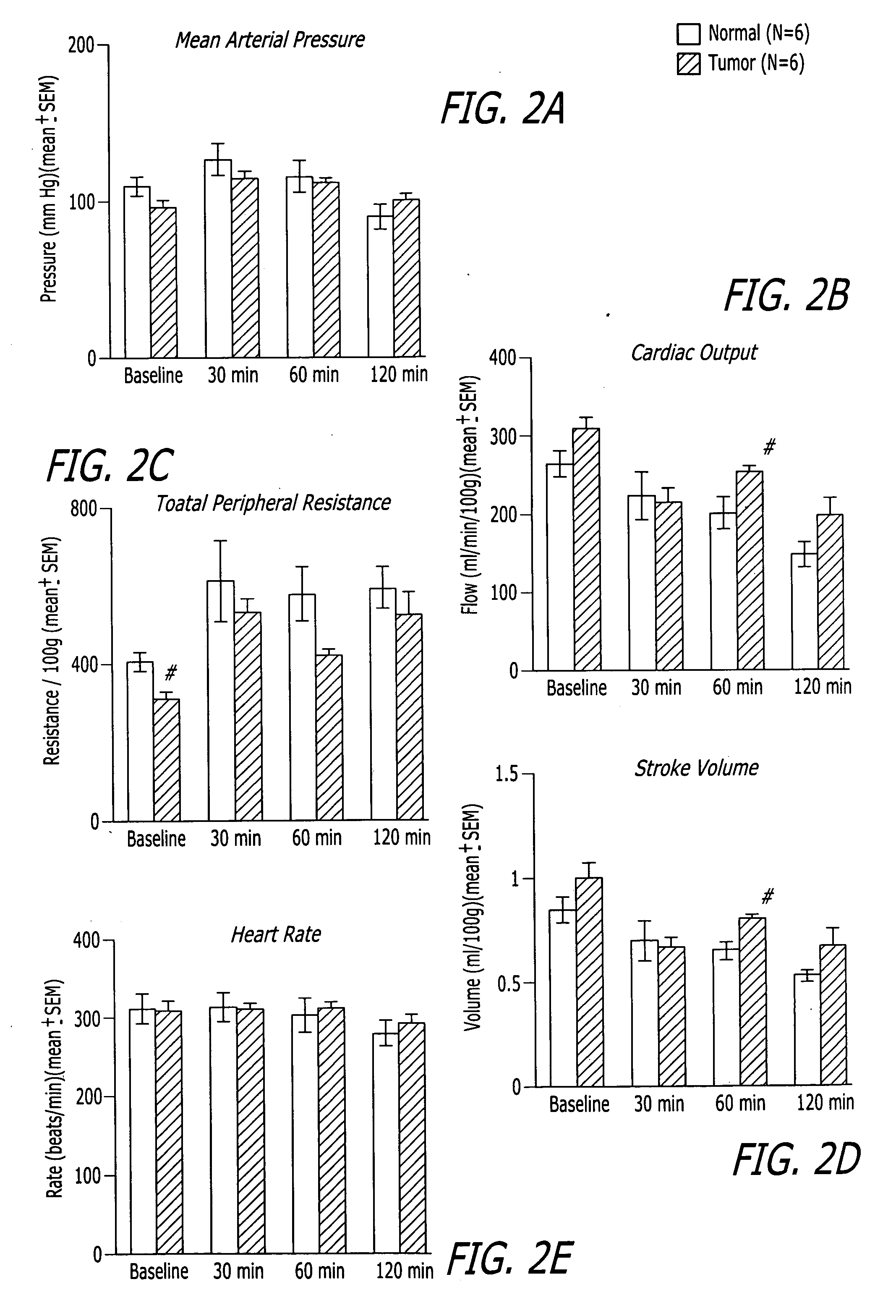
Methods, compositions and articles of manufacture for contributing to the treatment of solid tumors
ActiveUS20060211617A1Efficient deliveryConvenient treatmentHeavy metal active ingredientsBiocideAbnormal tissue growthAgonist
Methods, compositions and articles of manufacture for contributing to the treatment of a solid cancerous tumor are disclosed. The methods, compositions and articles of manufacture can utilize an endothelin B agonist (ETB) to enhance the delivery of a chemotherapeutic agent to a solid tumor in mammals, including humans.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Compositions and methods for modulation of vascular structure and/or function
InactiveUS20020019367A1Minimizing blood lossCessationBiocideSolution deliveryArteriolar VasoconstrictionVascular structure
The present invention relates to compositions comprising semi-crystalline beta-1-4-N-acetylglucosamine polymers (p-GlcNac) and methods utilizing such polymers modulation of vascular structure and / or function. The compositions and methods disclosed are useful for stimulating, in a p-GlcNac concentration-dependent manner, endothelin-1 release, vasoconstriction, and / or reduction in blood flow out of a breached vessel, as well as for contributing to or effecting cessation of bleeding. The methods of the present invention comprise topical administration of materials comprising semi-crystalline p-GlcNac polymers that are free of proteins, and substantially free of single amino acids as well as other organic and inorganic contaminants, and whose constituent monosaccharide sugars are attached in a beta-1-4 conformation.
Owner:MARINE POLYMER TECH
Method for isolated culture of human fat mesenchyma stem cell and special culture medium thereof
ActiveCN101314766AThe method of isolation and culture is simpleImprove efficiencySkeletal/connective tissue cellsAntigenMuscle injury
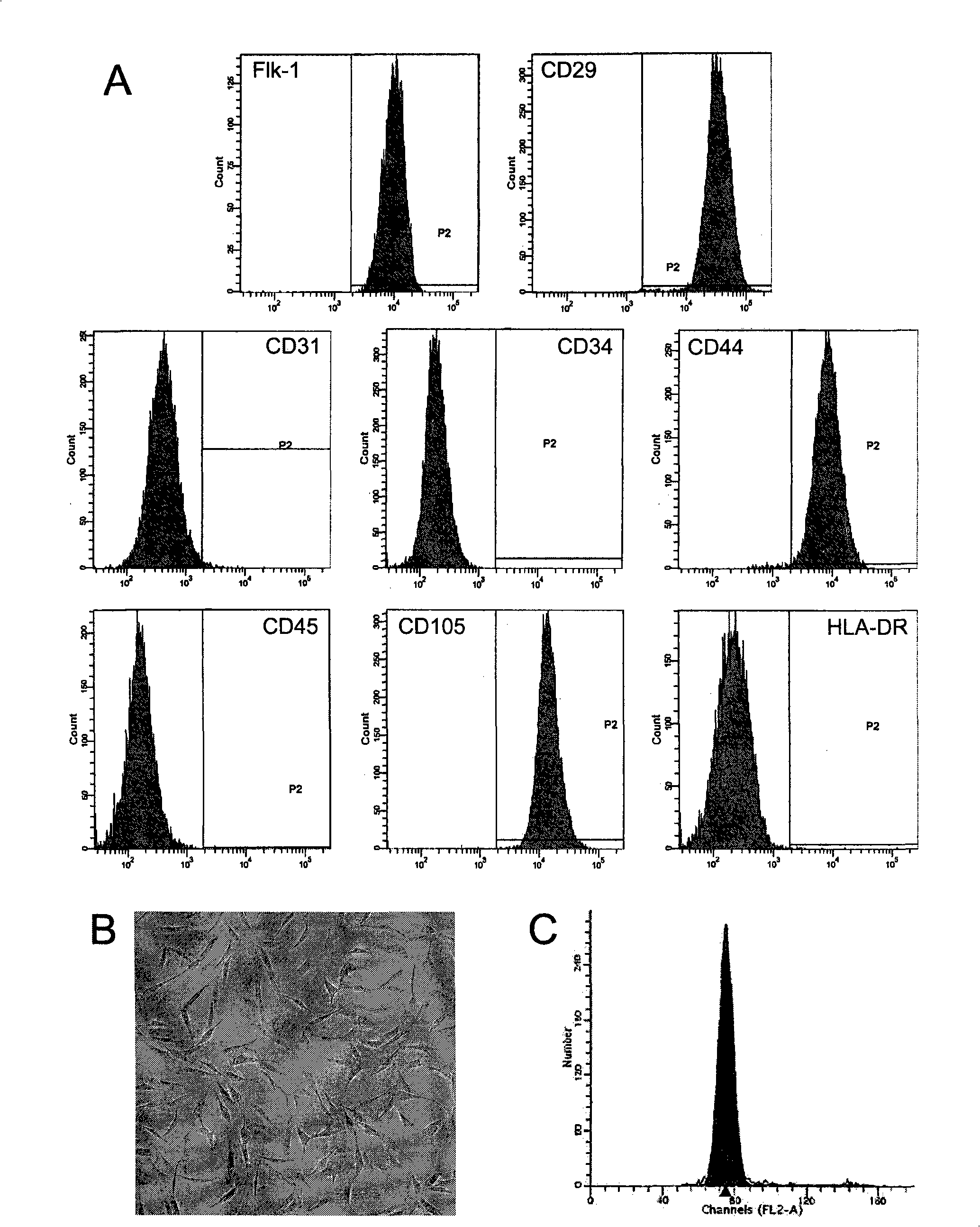
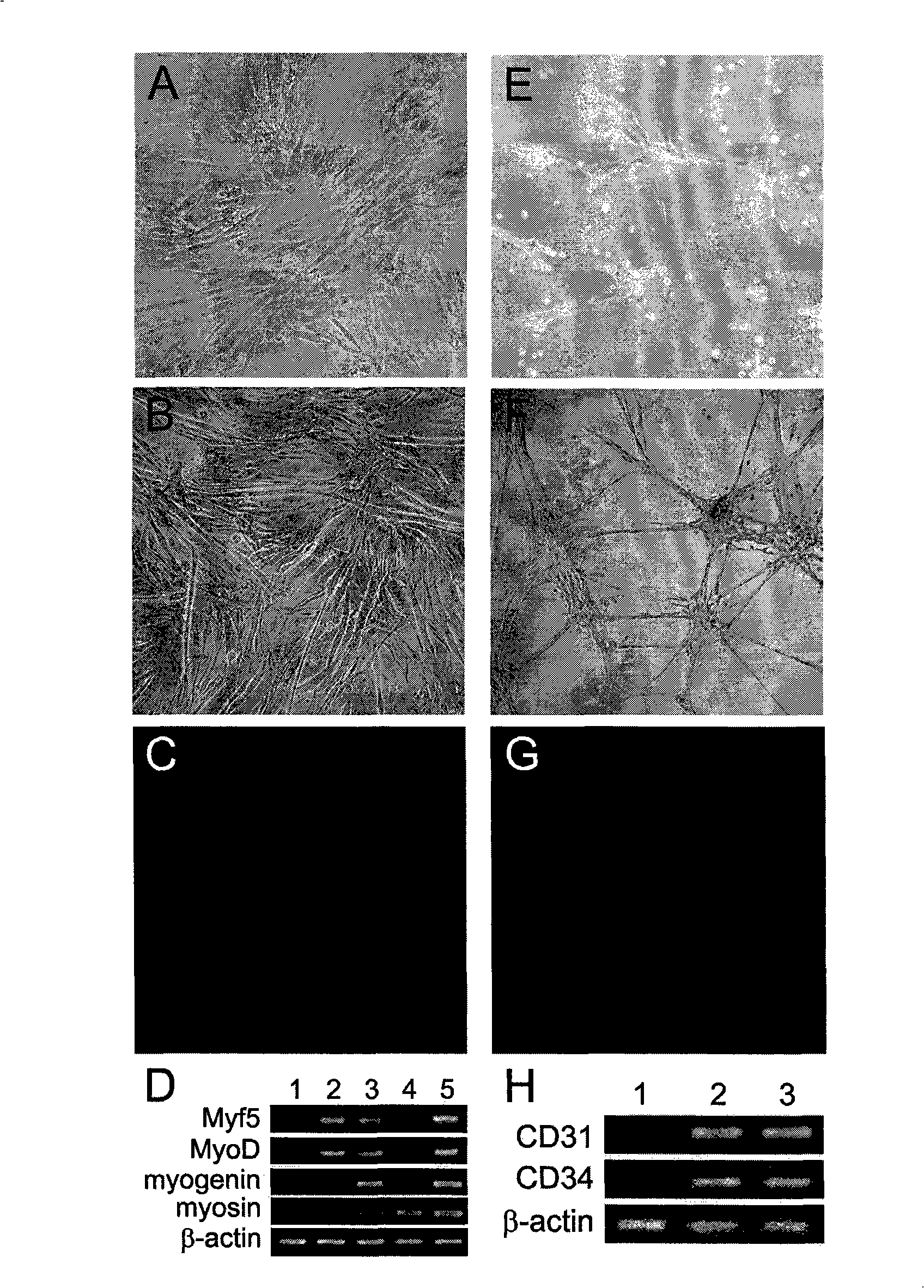
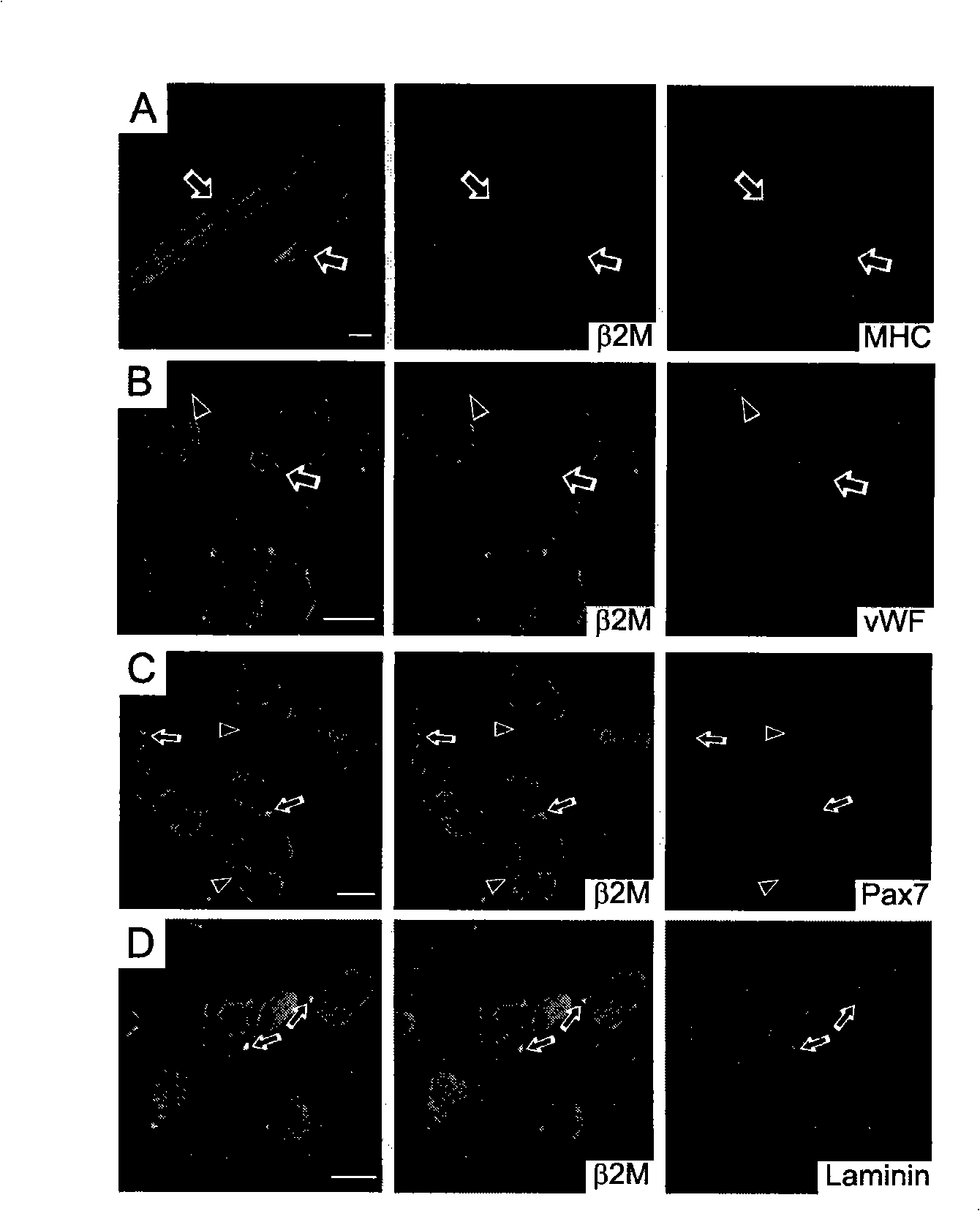
The invention discloses a method for separately culturing a human adipose mesenchymal stem cell and a dedicated culture medium thereof. The culture medium used for separately culturing the human adipose mesenchymal stem cell comprises an animal cell basic culture medium, fetal calf serum, an epidermal growth factor and a platelet-derived growth factor. The final concentration of the fetal calf serum is 1-200 mL / L, the final concentration of the epidermal growth factor is 1-100 ng / ml, and the final concentration of the platelet-derived growth factor is 1-100 ng / ml. The adipose mesenchymal stem cell of the invention has CD31-, CD34-, CD45- and HLA-DR-, as well as the phenotype of CD29+, CD44+, CD105+ and Flk-1+. The specificity cell surface marker and the relevant antihelion molecule of a skeletal muscle cell and a vascular endothelia cell can be expressed after inducement is performed in vitro. Muscle fiber, vascular endothelin and functional muscle satellite cells can be differentiated in a muscle injury model mouse body caused by medicine and the expression of dystrophin protein on the ducheme muscular dystrophy (DMD) model mouse (mdx) myolemma can be partially recovered, so as to release the pathological symptom of the model mouse.
Owner:微能生命科技集团有限公司
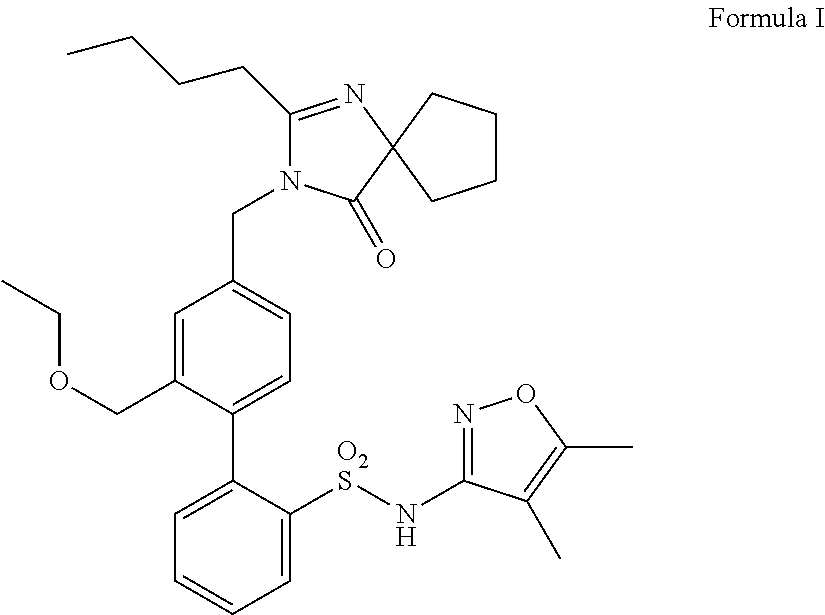
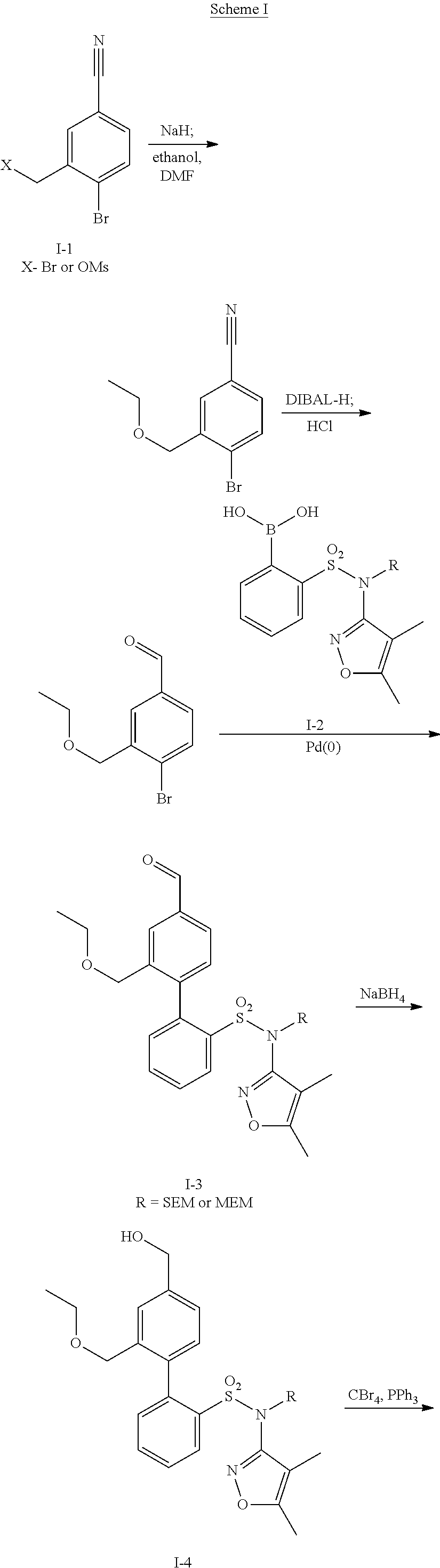
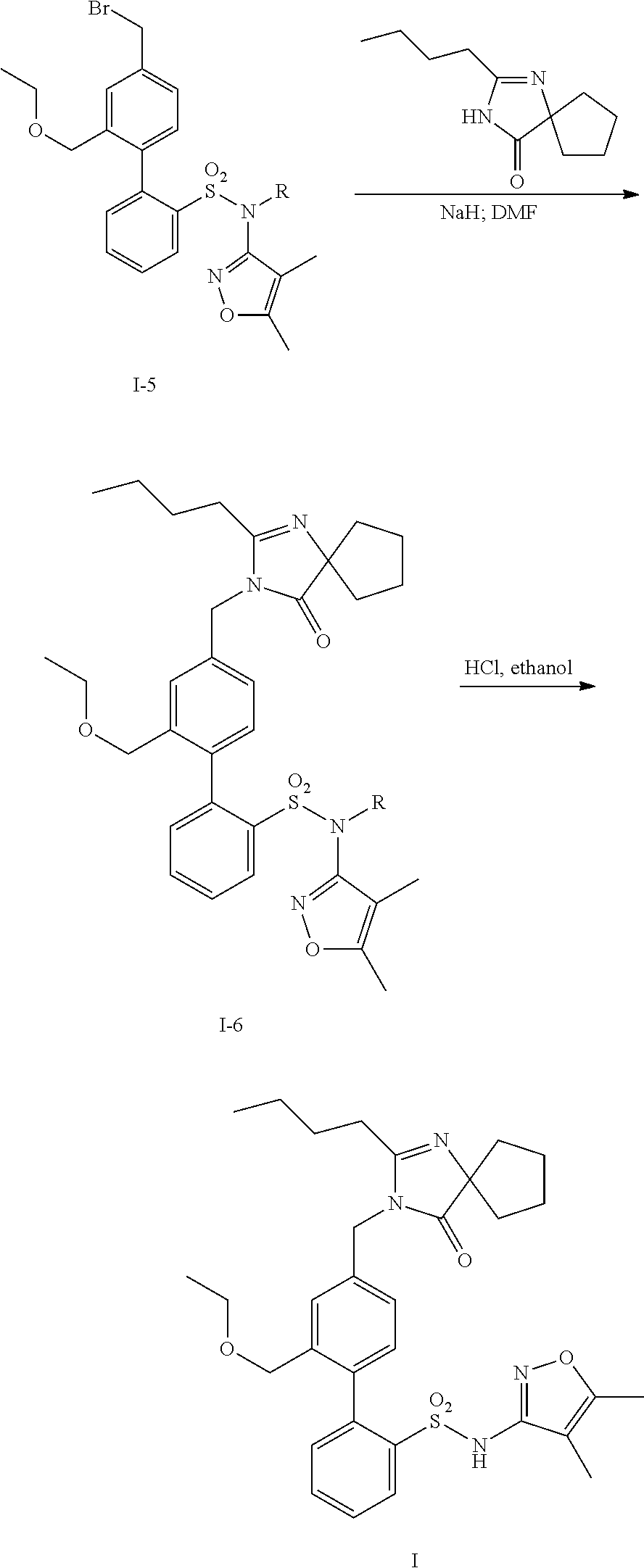
Oral formulations of diphenylsulfonamide endothelin and angiotensin ii receptor agonists to treat elevated blood pressure and diabetic nephropathy
InactiveUS20140142149A1Improves friabilityEasy to compressBiocideSenses disorderEndothelin receptor antagonistAgonist
Methods of administering and pharmaceutical compositions of a biphenyl sulfonamide compound which is a dual angiotensin and endothelin receptor antagonist are disclosed for treating diseases.
Owner:LIGAND PHARMA INC
Endothelin antagonists
Owner:ABBVIE INC
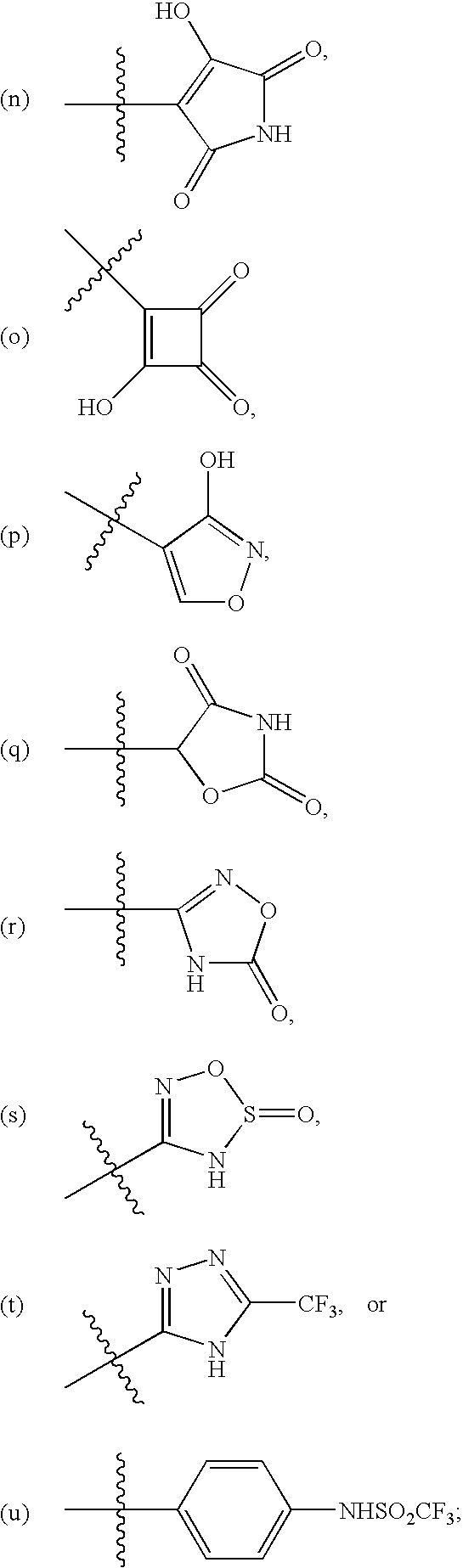
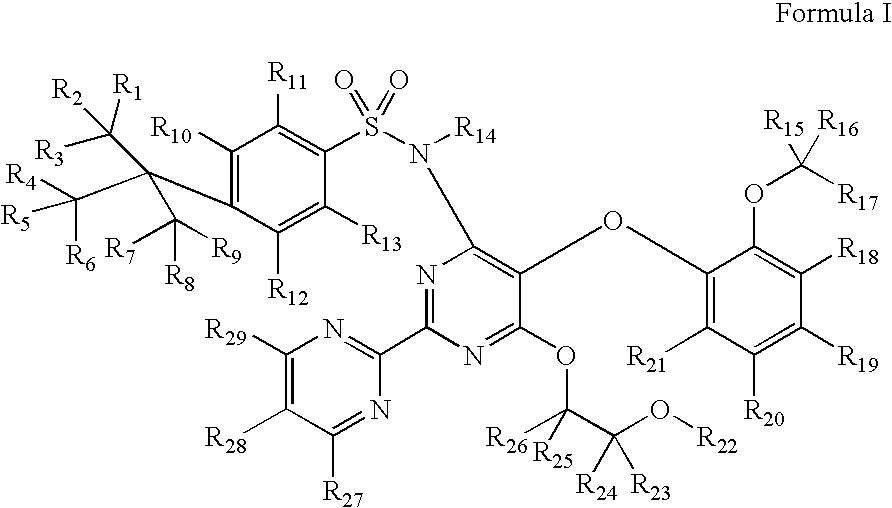
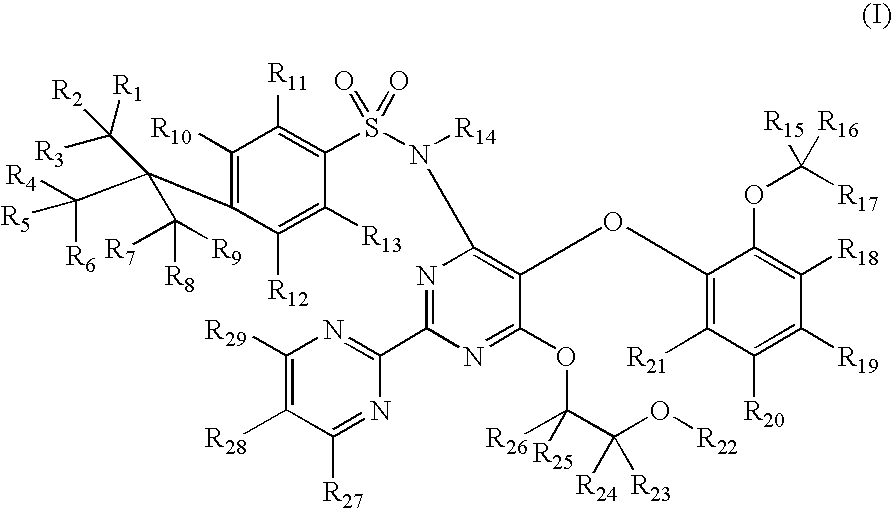
Substituted pyrimidines
InactiveUS20080242687A1Significant clinical effectOrganic active ingredientsBiocideEndothelinsMedicinal chemistry
Disclosed herein are substituted pyrimidine-based endothelin modulators of Formula I, processes of preparation thereof, pharmaceutical compositions thereof, and methods of use thereof.
Owner:AUSPEX PHARMA INC
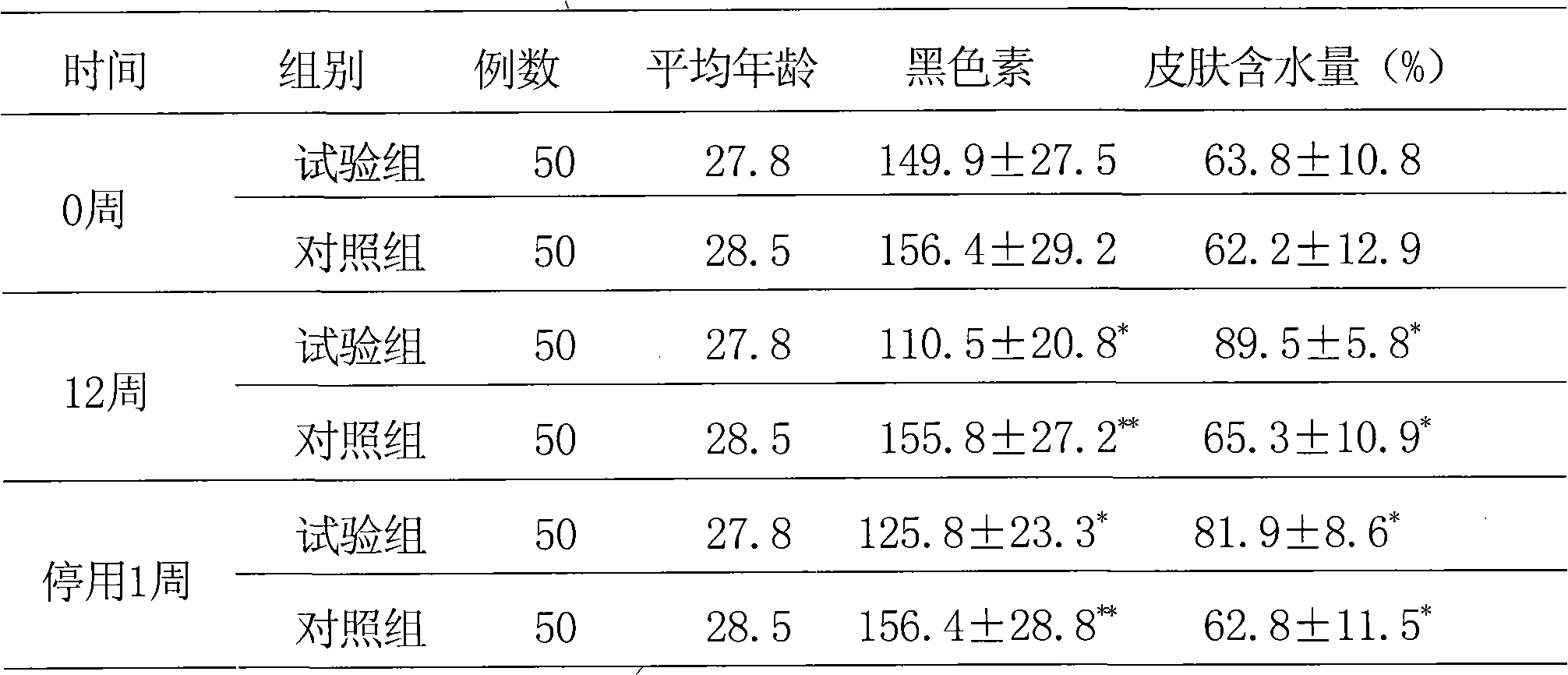
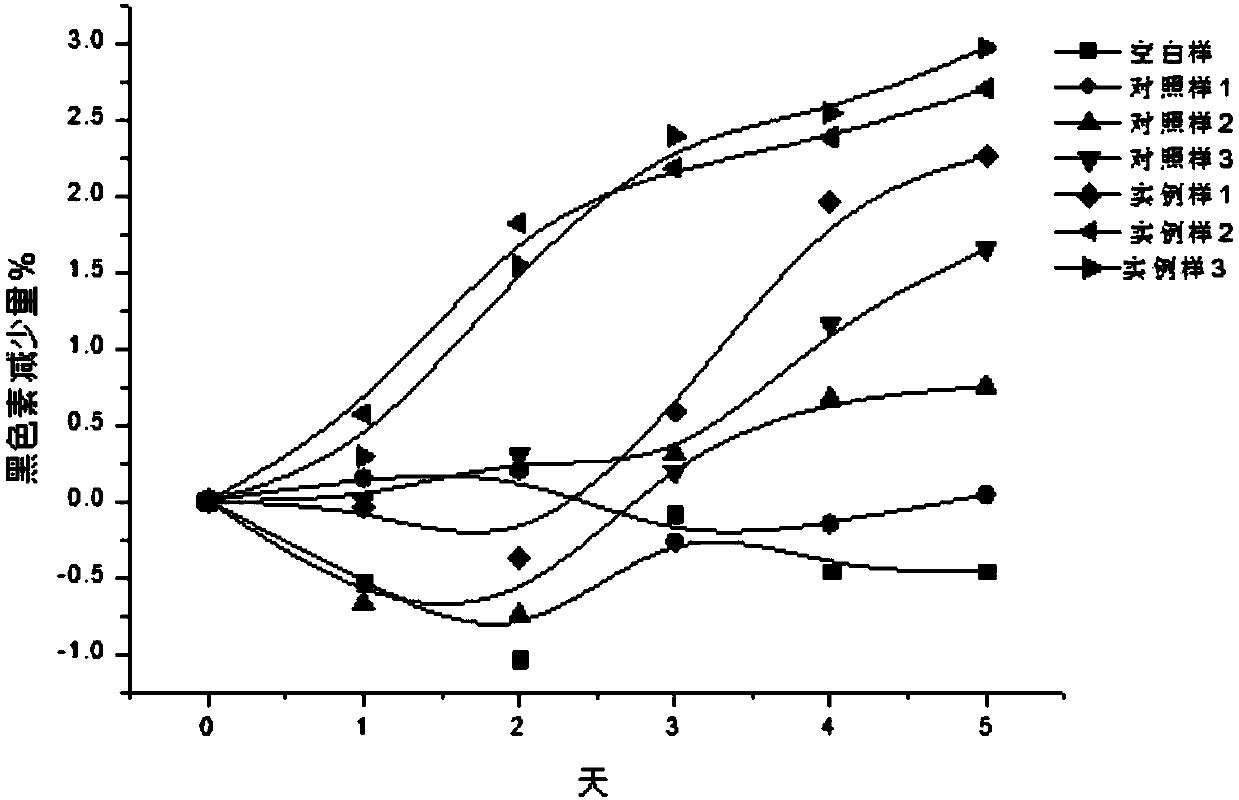
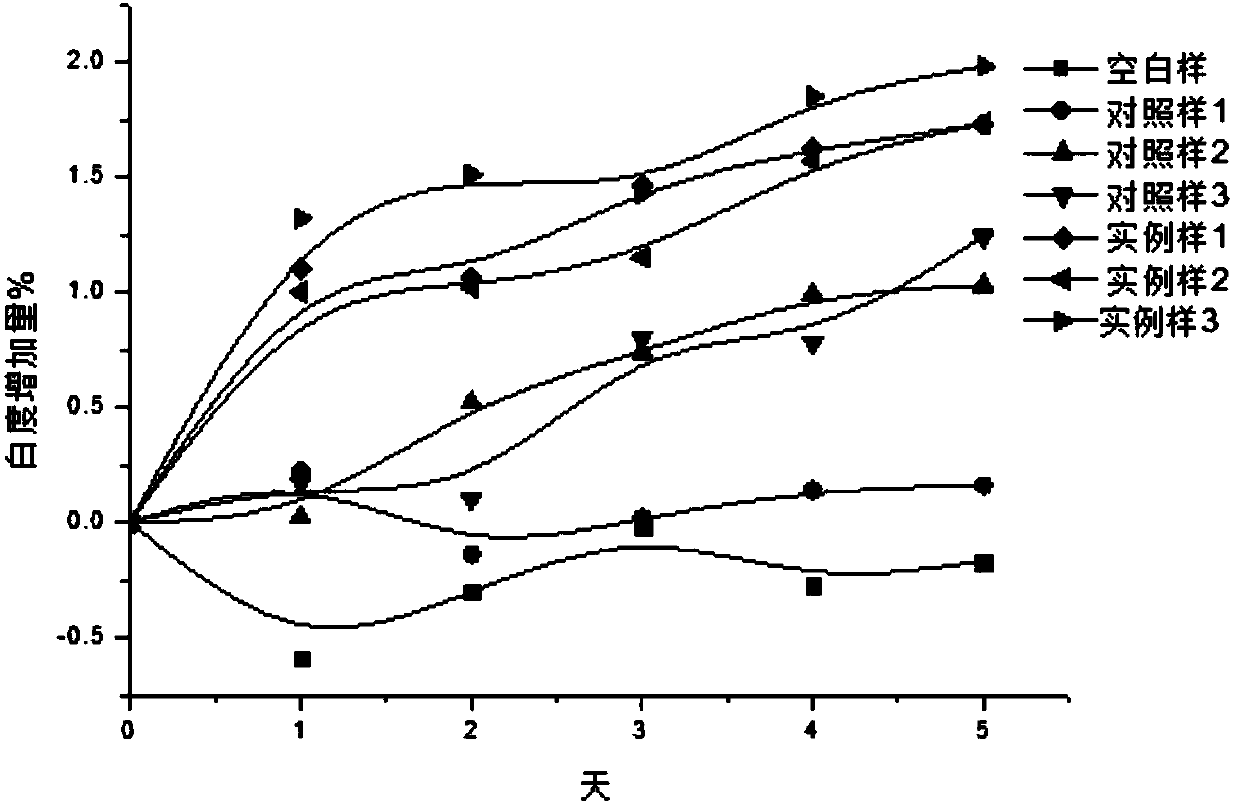
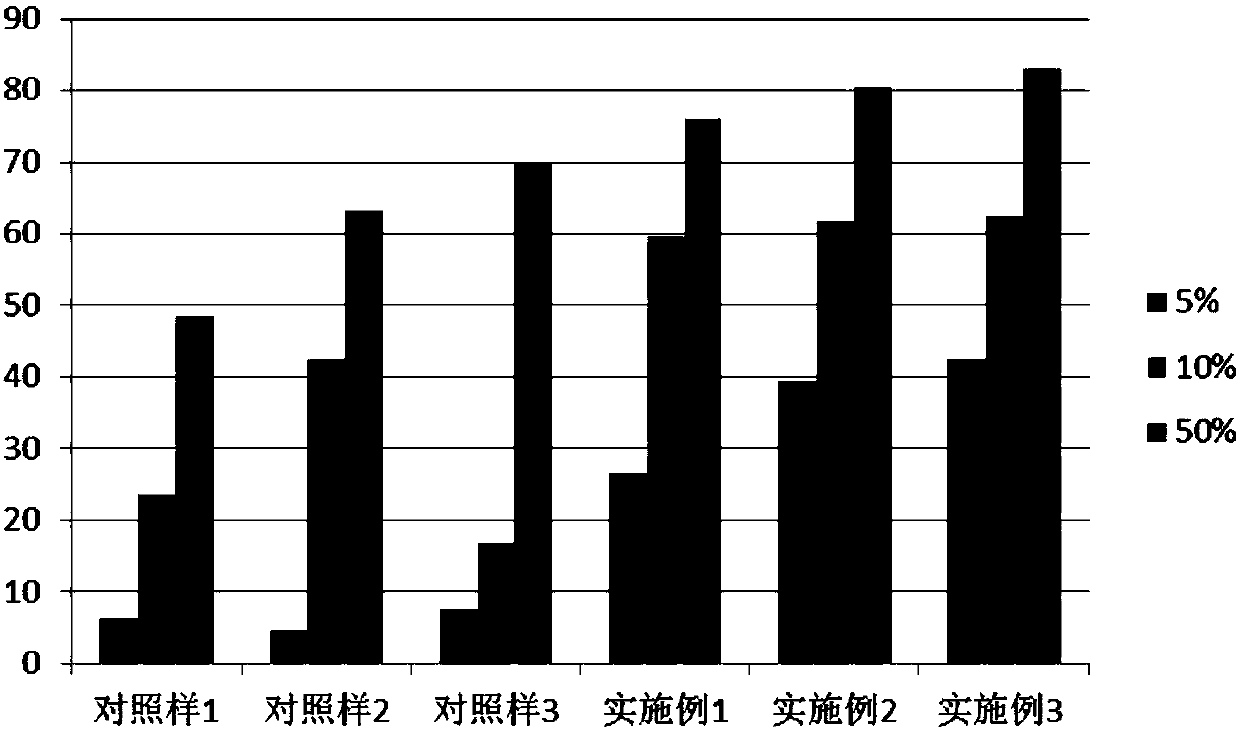
Composition of plant extracts and application in skin whitening and moisture preservation
InactiveCN101836944AStrong whitening and moisturizing effectNatural colorCosmetic preparationsToilet preparationsCentella asiatica extractSide effect
The invention relates to a composition of plant extracts and application thereof in skin whitening and moisture preservation. The composition comprises the following active ingredients in percentage by weight: 5 to 90 percent of green tea extract, 5 to 50 percent of ginkgo leaf extract and 5 to 45 percent of centella extract, wherein the composition accounts for 0.5 to 20 percent when applied to preparing external products of skin, and the composition is uniformly mixed with medically-acceptable excipients or carriers in proportion to prepare products in forms of gel, cream, serosity, aqueous solution agents, emulsions or solids. The composition has the effects on skin whitening and moisture preservation without adding moisture preserving components; compared with products with similar application, the composition has stronger effects on skin whitening and moisture preservation and smaller dosage; the composition is safe, has no toxic or side effect, has low allergy rates and inflammation probability, penetrates into skin dermis, does not change the normal apoptosis process of cells, restrains tyrosinase, dopachrome tautomerase, DHICA oxidase and endothelin appropriately and balances the metabolism of melanin, so that the color and luster of the skin is more natural; and in addition, the generated skin whitening effect is definite, and the composition is convenient to use in cosmetics.
Owner:杭州千岛湖康诺邦健康产品有限公司
Whitening composition and application thereof
InactiveCN107049880AImprove distributionEvenly distributedCosmetic preparationsToilet preparationsBenzoic acidInflammatory factors
The invention relates to a whitening composition (uniGlow) and an application thereof. The whitening composite comprises phenylbenzimidazole sulfonic acid, hydroxyphenylpropylamide benzoic acid, dipotassium glycyrrhizinate, undecylenoyl phenylalanine, hydrolyzed shellfish protein, beta-arbutin, ellagic acid, sodium ascorbate phosphate, carnosine, nicotinamide, inositol, papain and hesperidin extract. The whitening composition performs targeted inhibition from the melanin forming inducements of UV, inflammatory factors, melanoma and endothelin; in the melanin synthesis process, tyrosinase inhibits the reduction with a melanin intermediate, blocks the melanin transfer pathway, accelerates stratum corneum spalling and accelerates the metabolism; by conditioning, the skin is fine, tight, natural, white and bright.
Owner:HUNAN YUJIA COSMETICS MFG CO LTD
Oral formulations of diphenylsulfonamide endothelin and angiotensin ii receptor agonists to treat elevated blood pressure and diabetic nephropathy
ActiveUS20150164865A1Lower systolic blood pressureLower diastolic blood pressureBiocideSenses disorderDiseaseAngiotensin II receptor type 1
Methods of administering and pharmaceutical compositions of a biphenyl sulfonamide compound which is a dual angiotensin and endothelin receptor antagonist are disclosed for treating diseases.
Owner:LIGAND PHARMA INC
Method for orienting inducing and differentiating heart pacemaker cell by embryo source pluripotent stem cell
Cell transplantation for constructing biological heart pacemaker is used embryo source dry / ancestral cell and adult mesogalia dry cell as cell sources. The process is carried out by oriented differentiation inducing for heart pacemaker cell by embryo source multifunctional dry cell, bone marrow adhering to obtain primary culture cell, clone culturing to obtain purifying system by diluting, applying paracrine factor endothelin-1 or nervous adjusting protein-1, extracellular matrix laminated adherent protein and fiber connecting protein etc. substrate glue, trans-dyeing pacemaker gene HCN4, and in-vitro inducing embryo source multifunctional dry cell. It is various and non-immunogenicity. It can be used to treat sick sinus syndrome.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
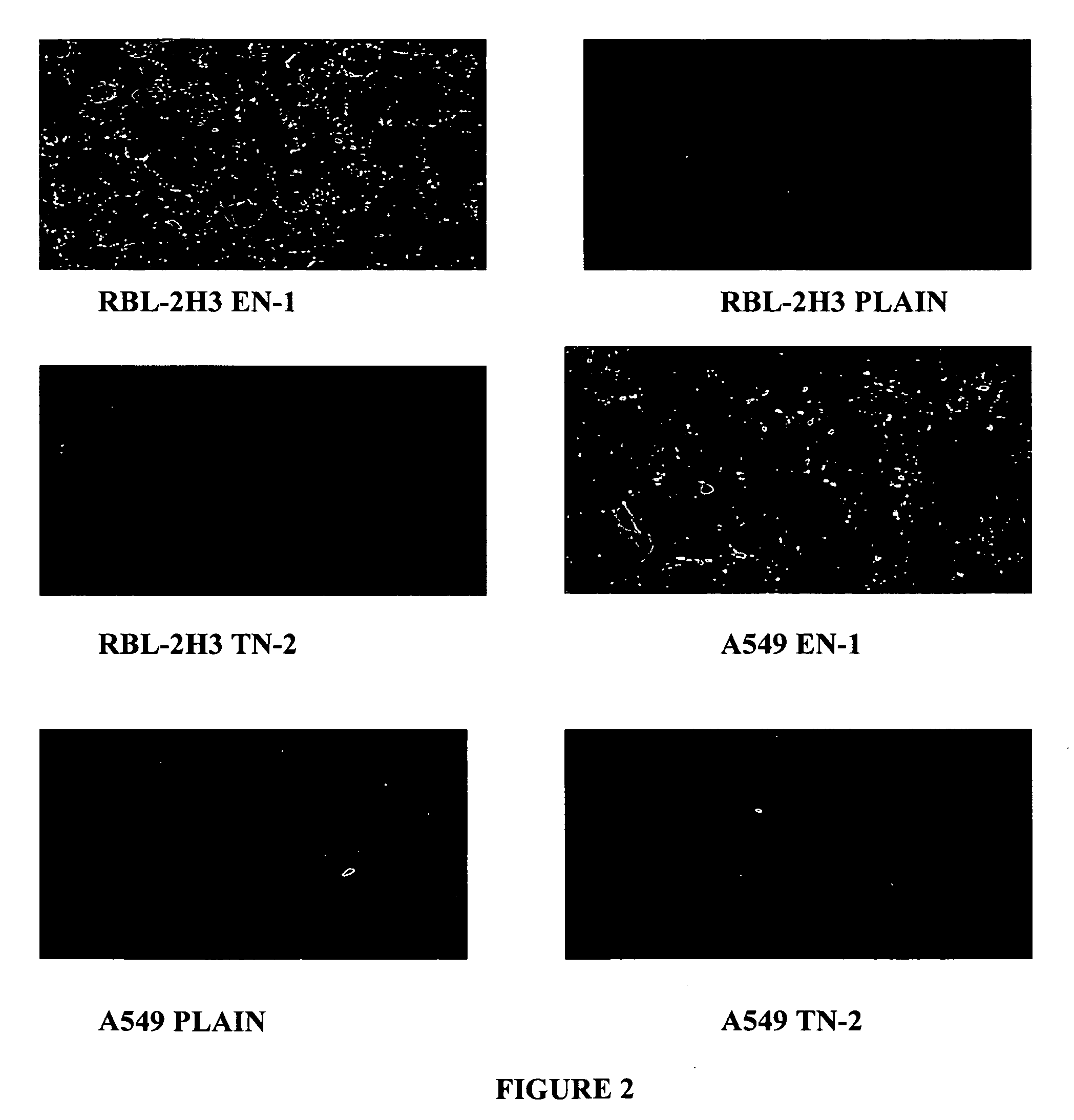
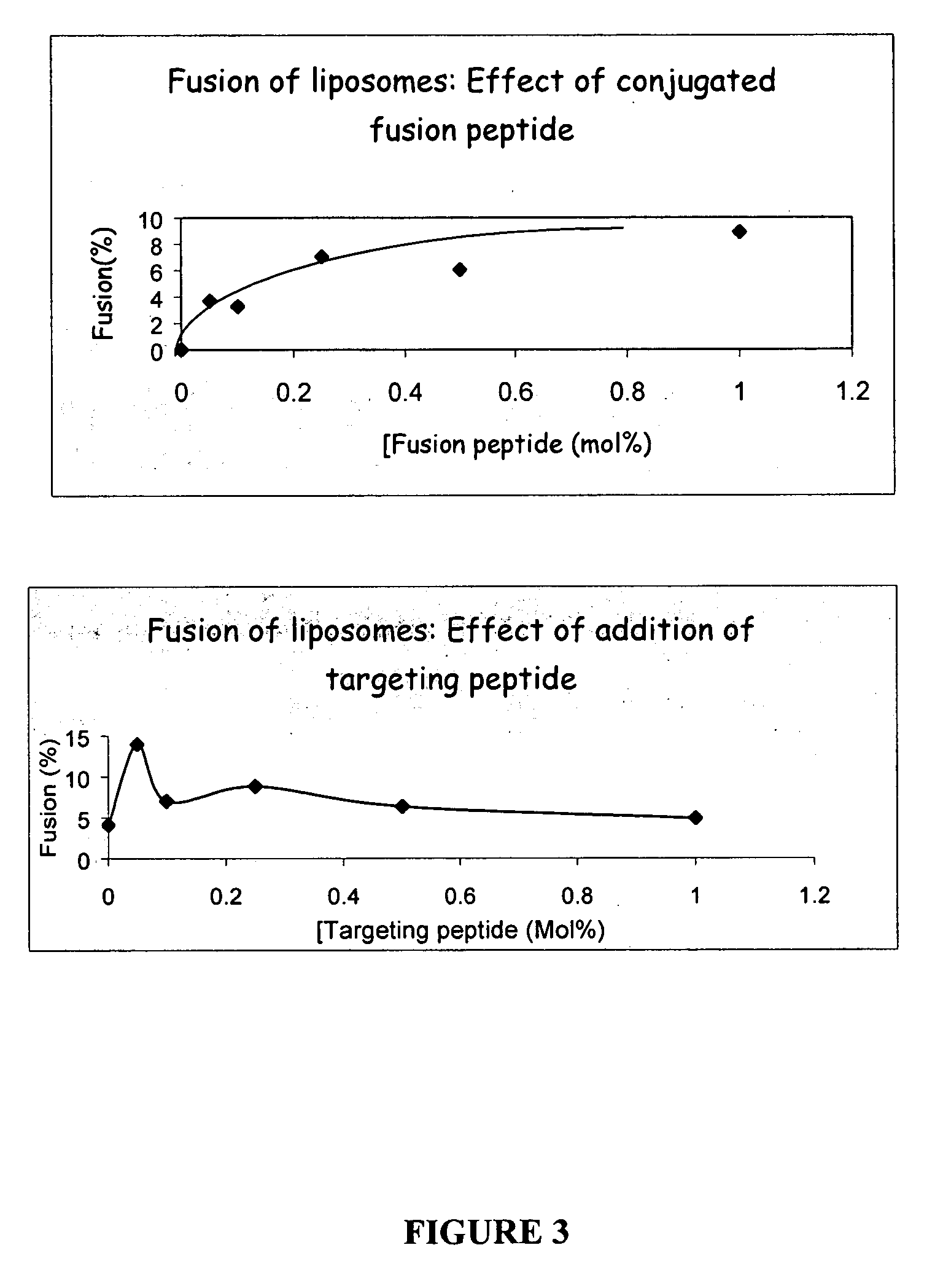
Liposomes containing novel targeting and/or fusogenic peptides, preparations containing them and therapeutic use thereof
InactiveUS20060240091A1SsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininTarget peptide
A novel targeting peptide from the C-terminal of endothelin and / or a novel fusogenic peptide from hemagglutinin are optionally conjugated to the carboxy group of 1,2-dioleoyl-sn-glycero-3-succinate and incorporated into liposomes for therapeutic treatment. The novel targeting peptide directs liposomes to lung cells, and, therefore, is useful for delivering liposomes encapsulating cholinesterase genes, particularly, the human serum butyryl cholinesterase (Hu BChE) gene, as a treatment against nerve agents. It is emphasized that this abstract is provided to comply with the rules requiring an abstract which will allow a searcher or other reader quickly to ascertain the subject matter of the technical disclosure. It is submitted with the understanding that it will not be used to interpret or limit the scope or meaning of the appended issued claims. 37 CFR §1.72(b).
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
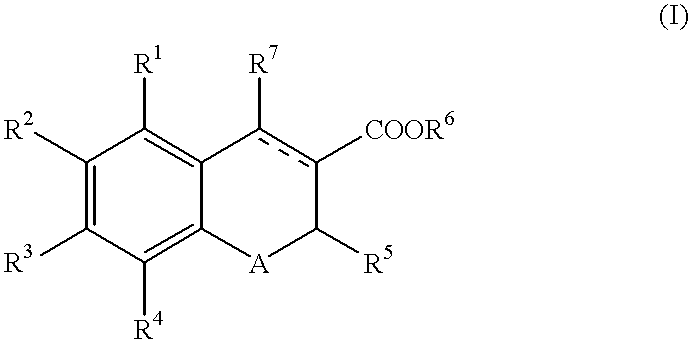
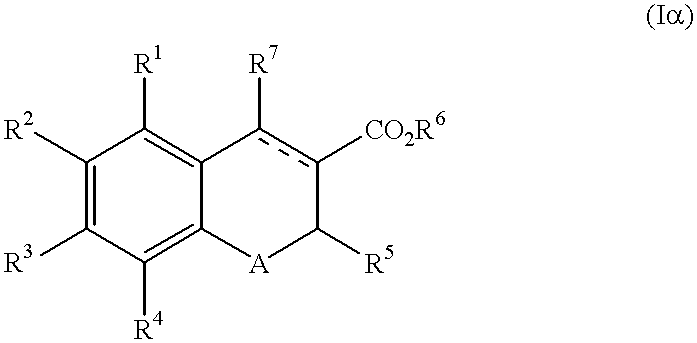
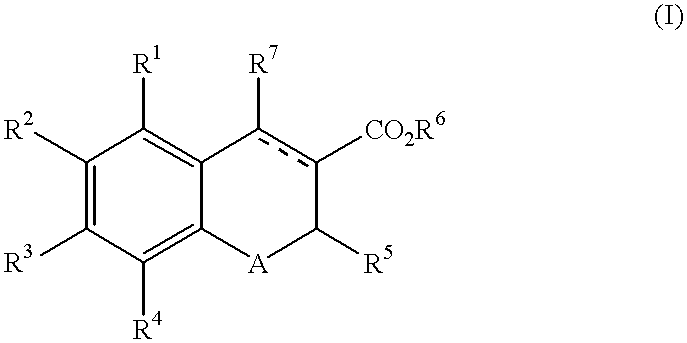
Chromene-3-carboxylate derivatives
The present invention provides a compound of the formula (I):wherein R1, R2, R3 and R4 are each independently hydrogen, optionally substituted alkyl, hydroxy, optionally substituted alkoxy or the like, R5 is optionally substituted alkyl, optionally substituted aryl, optionally substituted heterocyclic or the like, R6 is hydrogen, optionally substituted alkyl or the like, R7 is hydrogen, optionally substituted alkyl, optionally substituted alkoxy, optionally substituted aryl, optionally substituted heterocyclic or the like, A is S or O and a broken line represents presence or absence of a bond,pharmaceutically acceptable salt or hydrate thereof and a pharmaceutical composition or a pharmaceutical composition for use as an endothelin receptor antagonist, a peripheral circulation insufficiency-improving agent or a macrophage foam cell formation inhibitor comprising the compound.
Owner:SHIONOGI & CO LTD
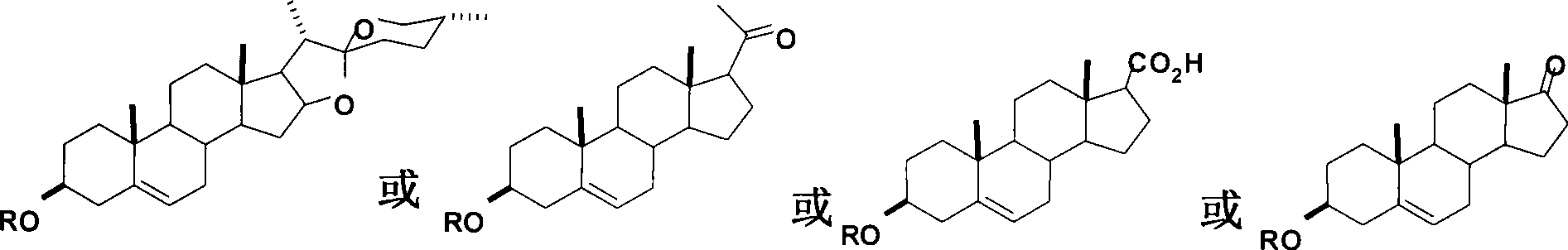
Steroid compounds with endothelin receptor A antagonistic activity and intermediate thereof
InactiveCN101475623AHas endothelin receptor A antagonistic activityOrganic active ingredientsSteroidsSapogeninKetone
The invention provides a structure and a synthetic method thereof. The structure is obtained by modifying structures such as yam steroid sapogenin, proluton, dehydroepihydro-ketone and the like, and has an endothelin receptor A antagonistic active compound.
Owner:JIANGSU SIMCERE PHARMACEUTICAL R & D CO LTD
Methods, compositions and articles of manufacture for contributing to the treatment of cancers
InactiveUS20070032422A1Efficient deliveryConvenient treatmentHeavy metal active ingredientsBiocideAgonistOncology
Methods, compositions and articles of manufacture for contributing to the treatment of cancers, including solid tumors, are disclosed. The methods, compositions and articles of manufacture can utilize an endothelin B agonist (ETB) to enhance the delivery and resulting efficacy of a chemotherapeutic agent.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS +1
Depilatories and agents for external use
InactiveUS6884772B1Reduce in quantityGood effectCosmetic preparationsHormone peptidesTopical preparationAgonist
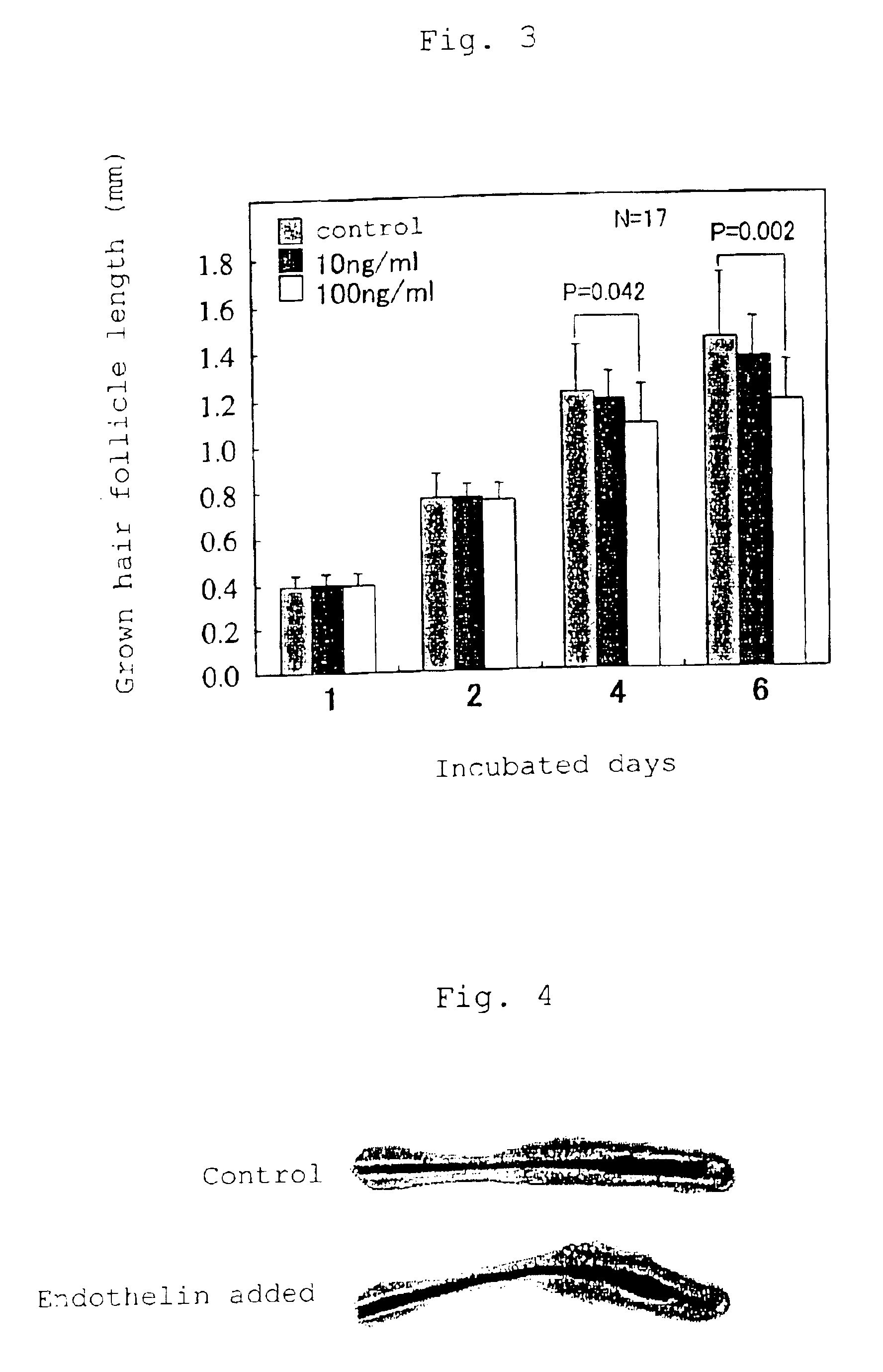
This invention relates to depilatories and external preparations, each of which contains endothelin or its agonist, and also to a depilating method characterized by administering endothelin or its agonist.As the depilatories and external preparations according to the present invention inhibit growth of hair in mammalian skin, their application to the human body as drugs or cosmetics permits safe and effective removal of hair from the body. These depilatories and external preparations are also usable as wool harvesting preparations.
Owner:KAO CORP
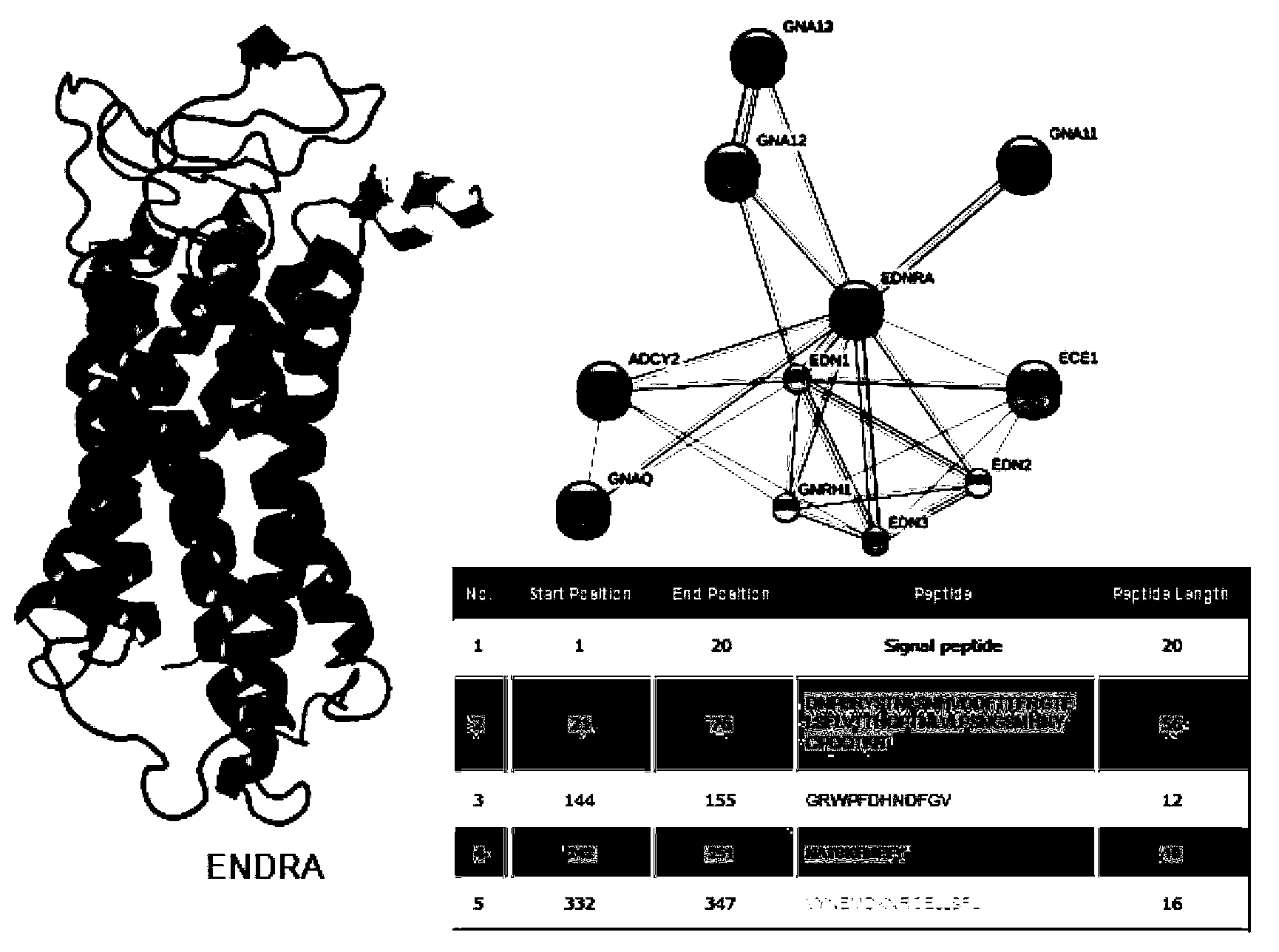
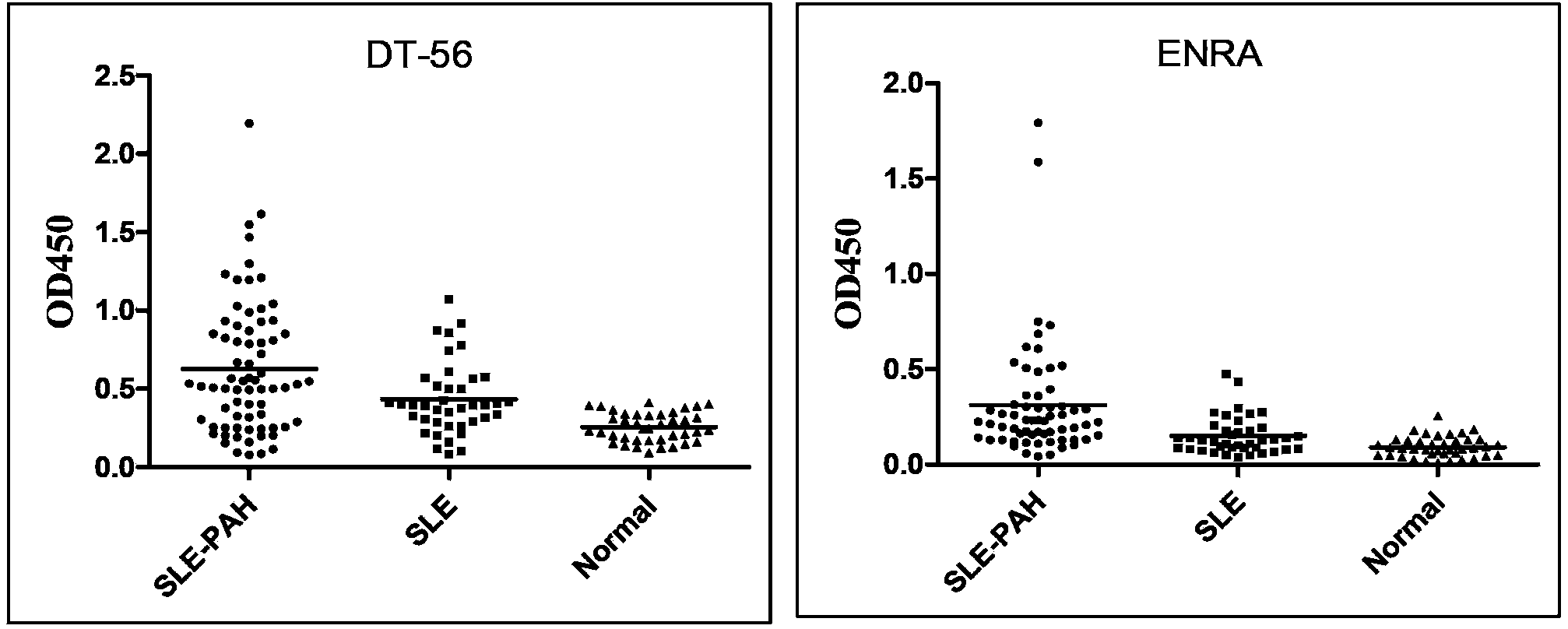
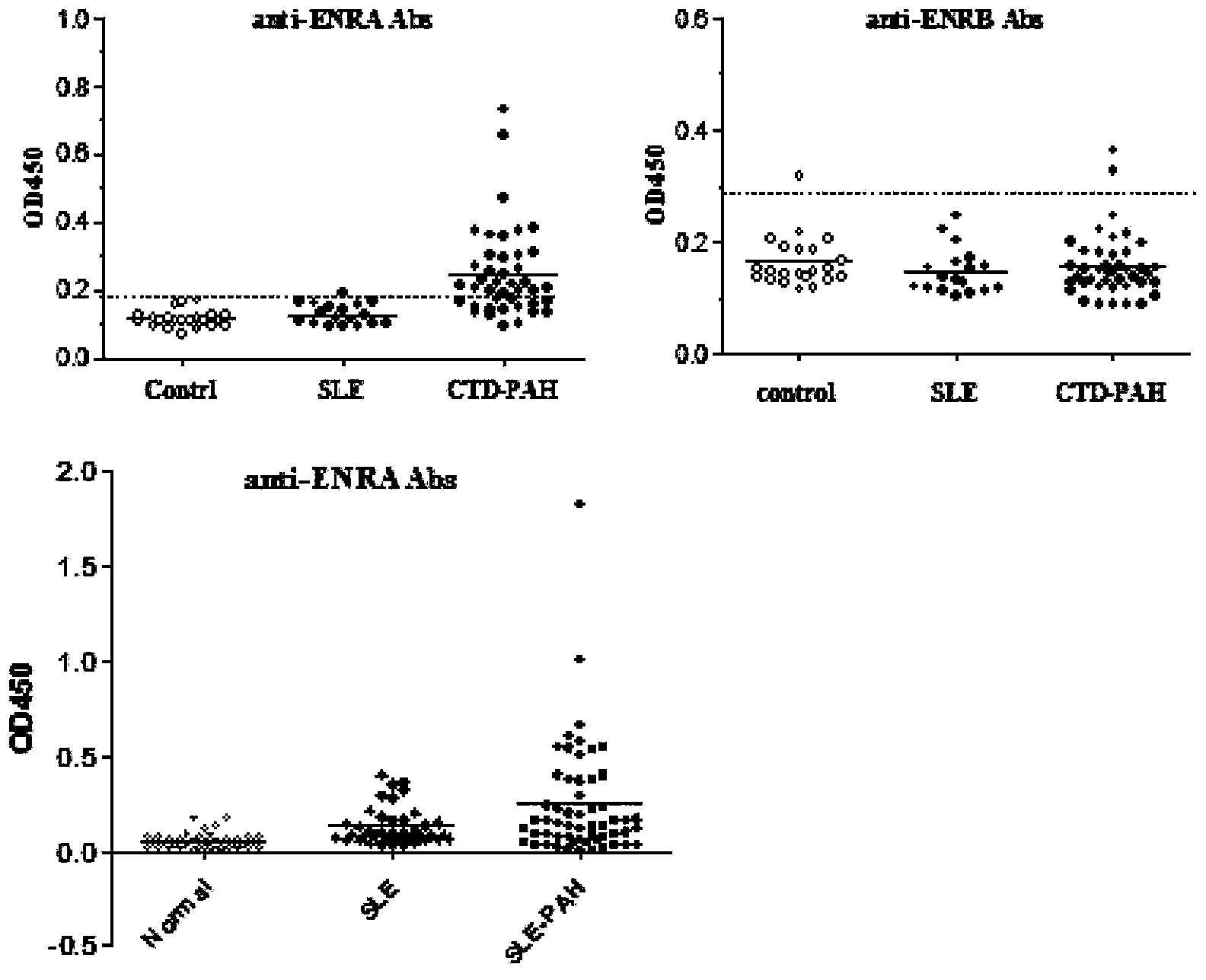
Enzyme-linked immunosorbent assay (ELISA) based on anti-ENRA (anti-endothelin receptor A) antibody of epitope antigen peptide and application thereof in CTD-PAH (connective tissue diseases-pulmonary arterial hypertension)
InactiveCN103728454ALow costImproving the practicality of clinical testingDisease diagnosisBiological testingAmino acidPulmonary hypertension
The invention relates to an enzyme-linked immunosorbent assay (ELISA) based on anti-ENRA (anti-endothelin receptor A) antibody of epitope antigen peptide; the enzyme-linked immunosorbent assay (ELISA) can be used in clinical tests of CTD-PAH (connective tissue diseases-pulmonary arterial hypertension); four extracellular peptide fragments with different lengths are synthesized in vitro, an epitope peptide fragment in good consistency with full-length ENRA is screened, the epitope peptide fragment is artificially synthesized to use as an antigen peptide package board to establish the enzyme-linked immunosorbent assay (ELISA) based on the anti-ENRA (anti-endothelin receptor A) antibody of the epitope antigen peptide; the epitope antigen peptide is a peptide fragment comprising the following amino acid sequence: DNPERYSTNLSNHVDDFTTFRGTELSFLVTTHQPTNLVLPSNGSMHNYCPQQTKIT; the enzyme-linked immunosorbent assay reduces the cost using full-length eukaryotic-expression endothelin receptor as a substrate, improves the clinical detection practicality, becomes a biomarker of CTD-PAH (especially SLE(systemic lupus erythematosus)-PAH), and provides valuable information for clinical diagnosis and treatment decisions.
Owner:吴庄民
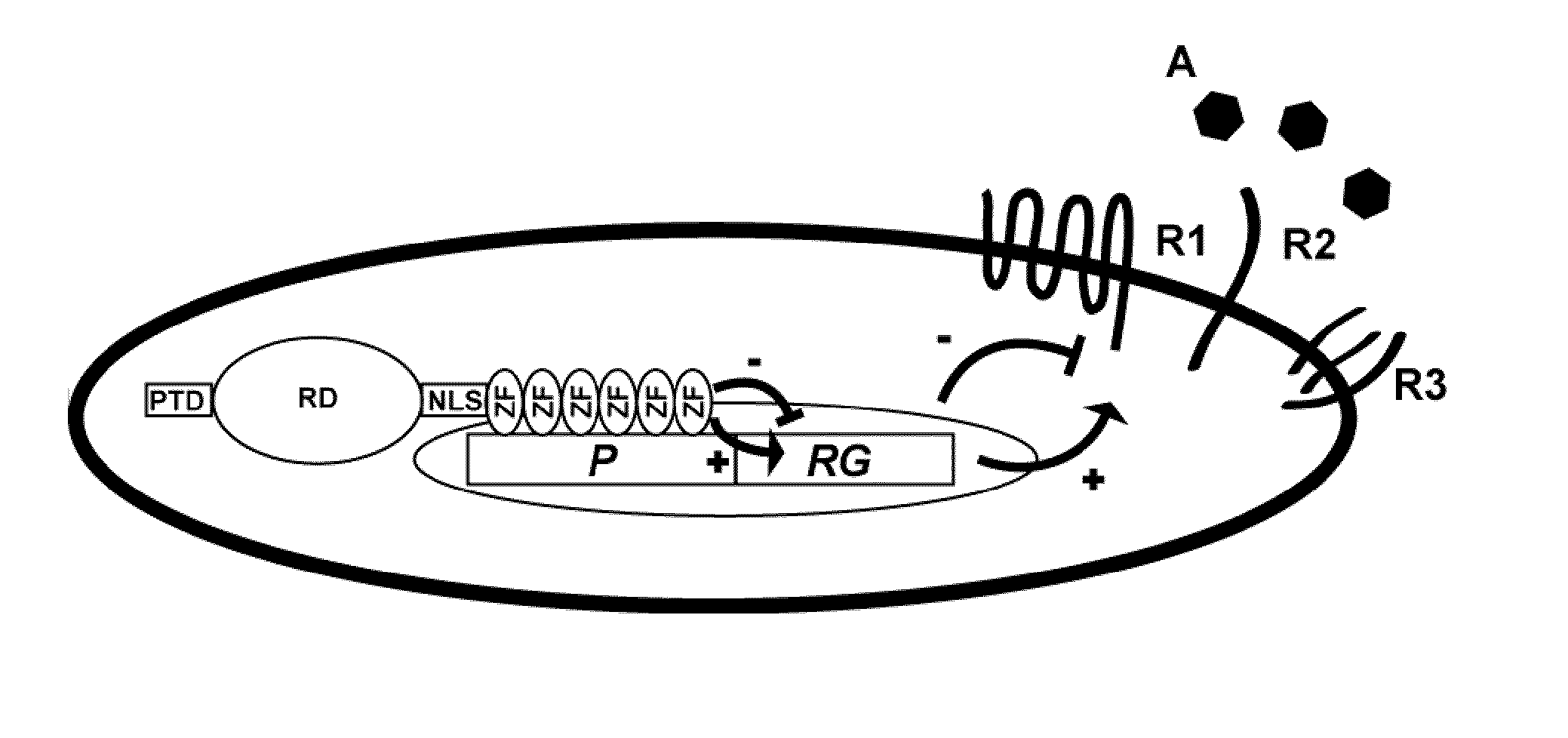
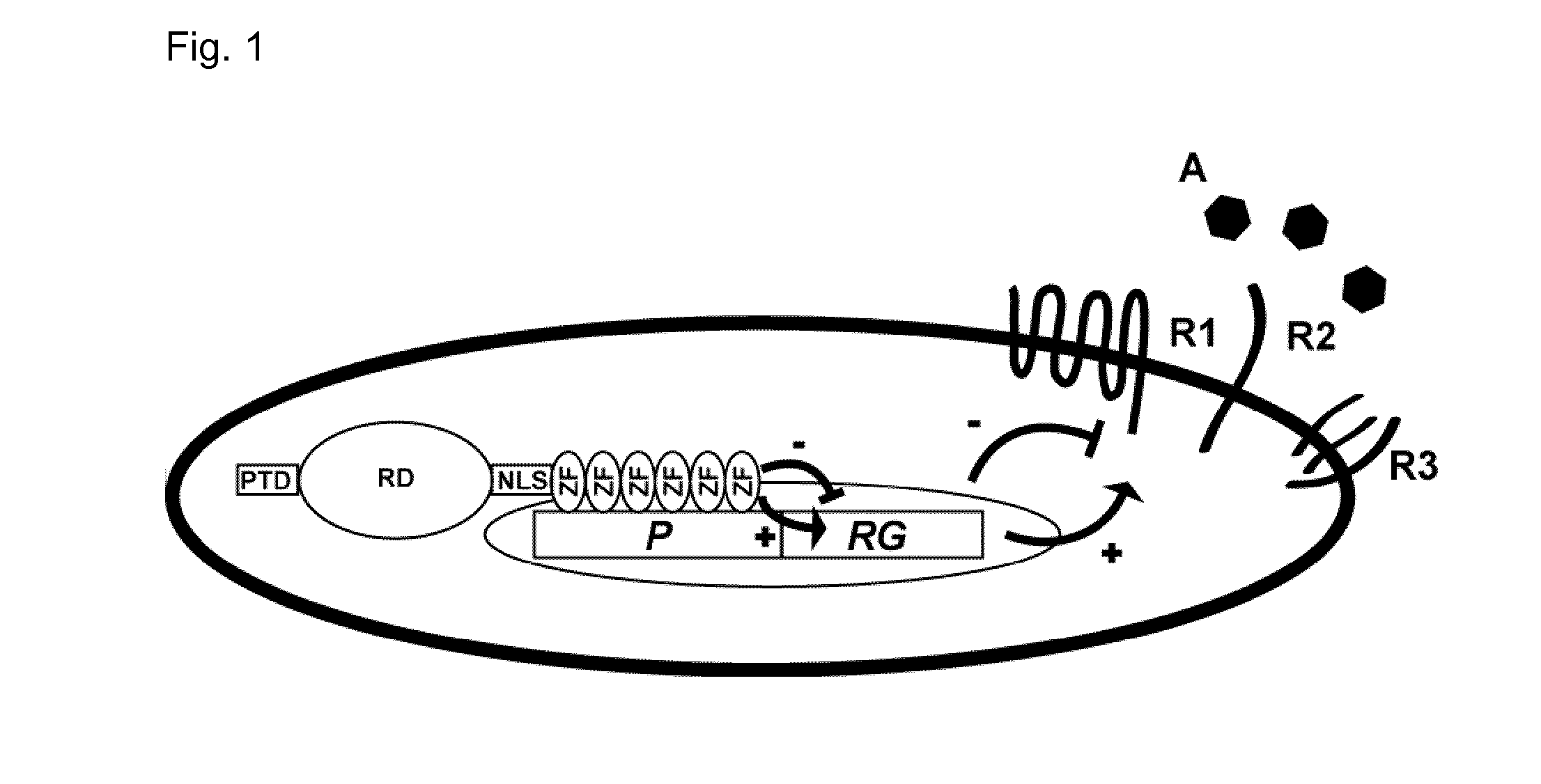
Regulation of receptor expression through delivery of artificial transcription factors
The invention relates to an artificial transcription factor comprising a polydactyl zinc finger protein targeting specifically a receptor gene promoter fused to an inhibitory or activatory protein domain, a nuclear localization sequence, and a protein transduction domain. In particular examples these receptor gene promoters regulate the expression of the endothelin receptor A, the endothelin receptor B, the Toll-like receptor 4 or the high-affinity IgE receptor. Artificial transcription factors directed to the endothelin A or B receptors are useful in the treatment of diseases modulated by endothelin, such as cardiovascular diseases, and, in particular, eye diseases, e.g. retinal vein occlusion, retinal artery occlusion, macular edema, optic neuropathy, central serous chorioretinopathy, retinitis pigmentosa, Leber's hereditary optic neuropathy, and the like. Artificial transcription factors directed to the Toll-like receptor 4 or the IgE receptor are useful for the treatment of autoimmune disorders, and the like, and allergic disorders, respectively.
Owner:ALIOPHTHA
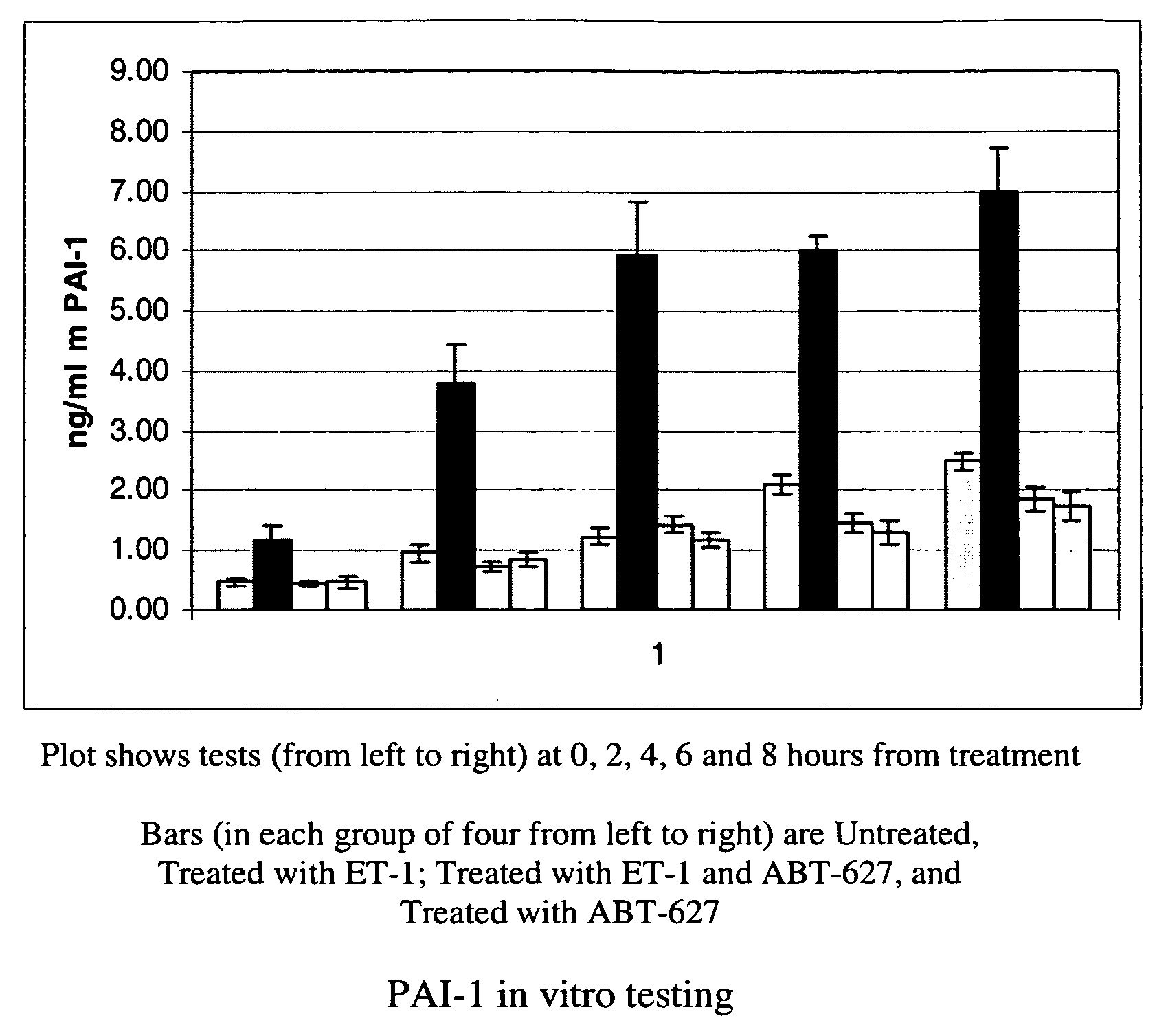
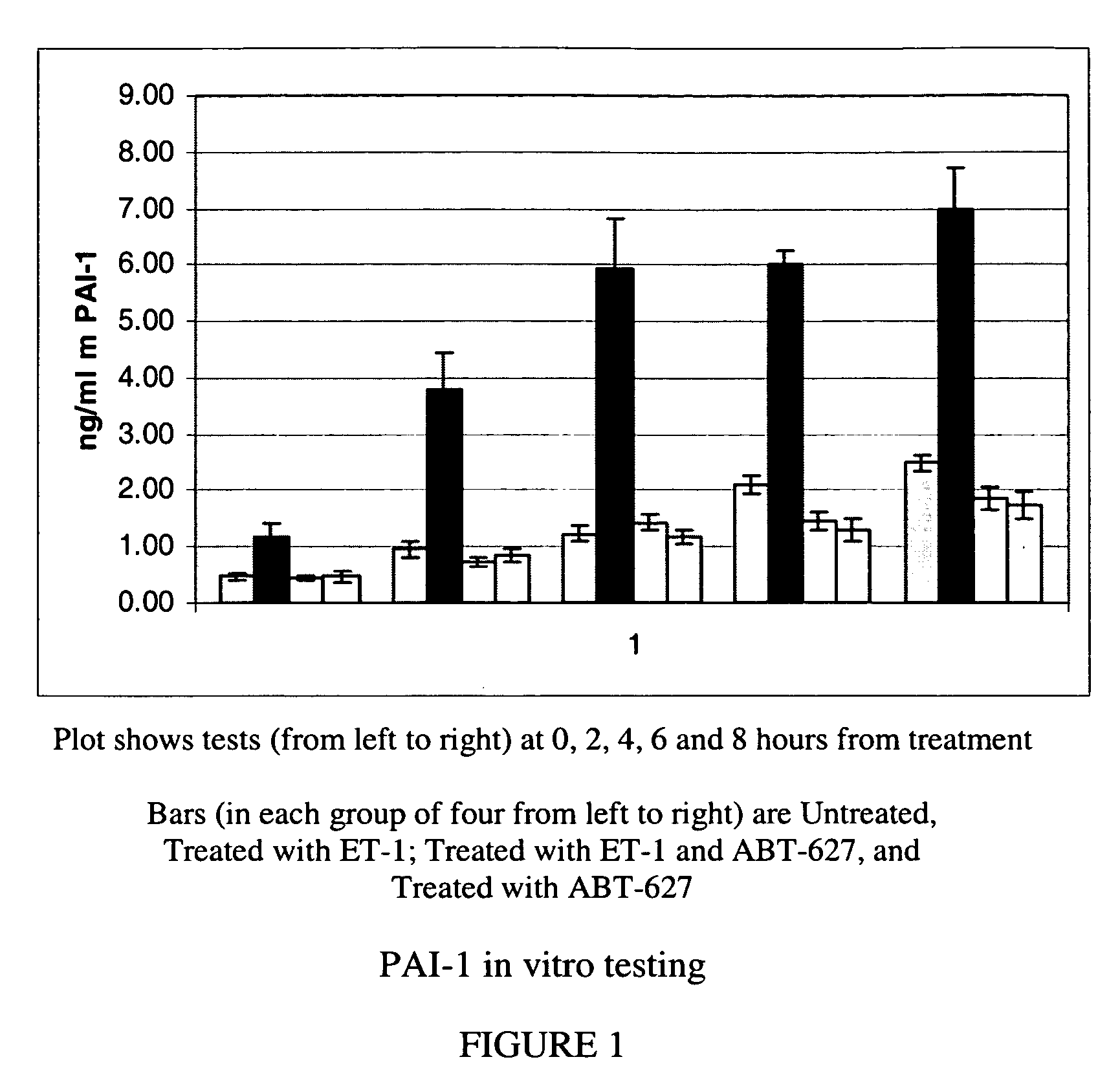
Companion diagnostic assays for endothelin receptor antagonists
InactiveUS20080102451A1Increase choiceMicrobiological testing/measurementBiological testingMedicineTissue sample
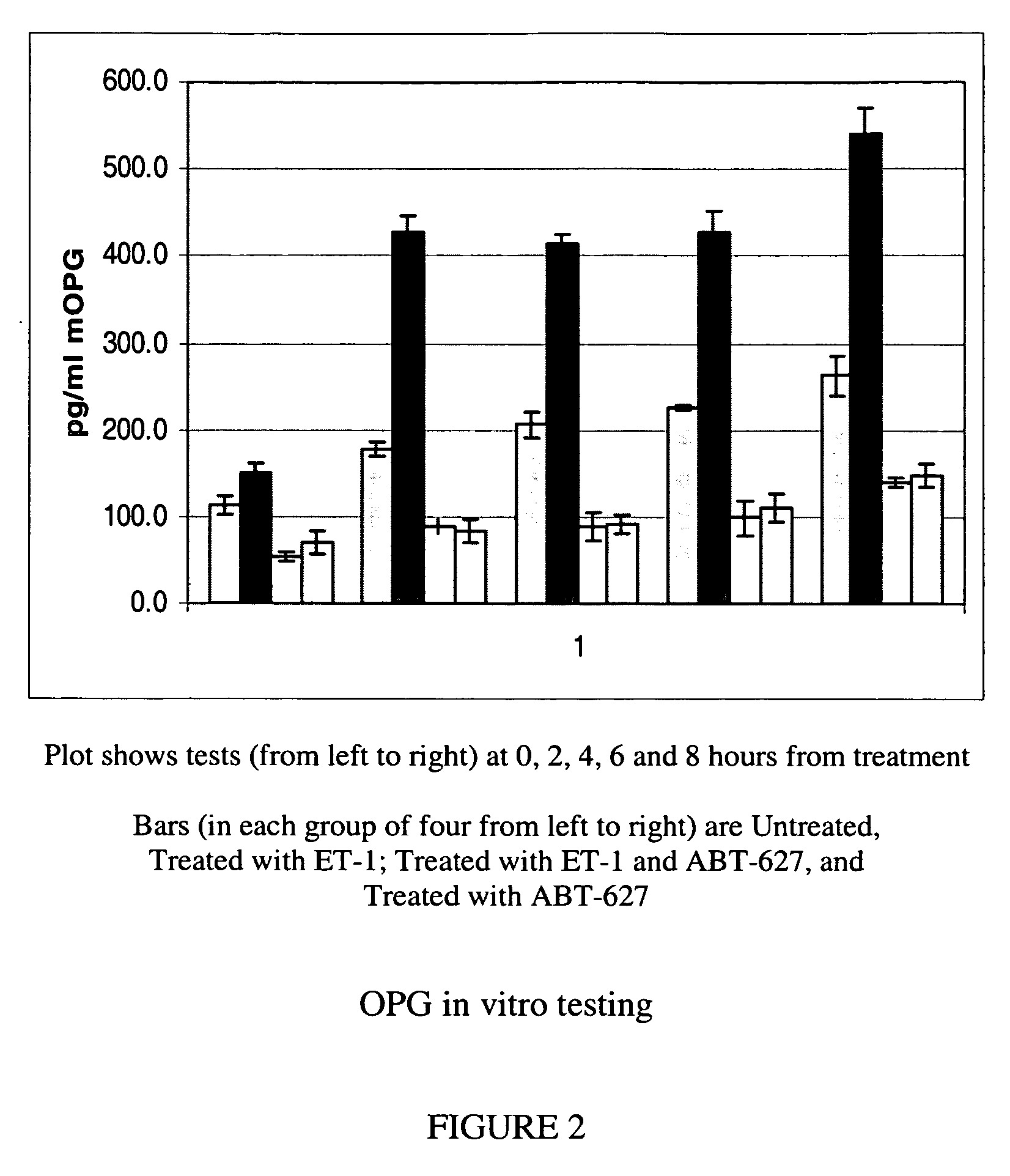
Methods for identifying cancer patients eligible to receive endothelin receptor antagonist therapy and for monitoring patient response to endothelin receptor antagonist therapy comprise assessment of the expression levels of at least one of PAI-1, uPA, TGFbeta2, IL-6, IL-8 and OPG in a patient tissue sample. The methods of the invention allow more effective identification of patients to receive endothelin receptor antagonist therapy and of determination of patient response to the therapy.
Owner:ABBOTT LAB INC
N-substituted benzothiophenesulfonamide derivatives
The present invention relates to an N-substituted benzothiophenesulfonamide derivative or a pharmaceutically acceptable salt thereof and applications thereof. Furthermore, it provides an agent for preventing or treating cardiac or circulatory disease and so on caused by abnormal increase of production of angiotensin II or endothelin I based on chymase activity, or by activation of mast cell, and an agent for preventing adhesion after surgery, wherein the agent has a selective inhibitory action on chymase.
Owner:TOAEIYO
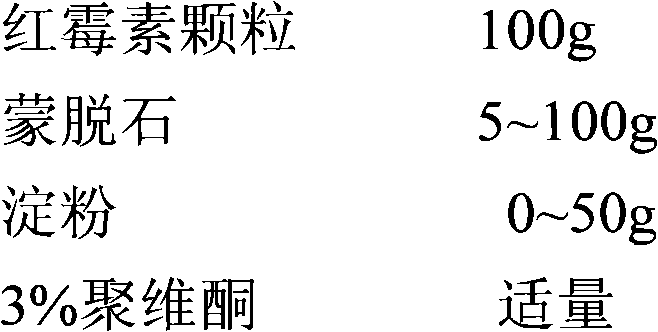
New preparation of erythrocin and relevant drug thereof and preparation method of new preparation
The invention relates to a preparation method of new preparation of erythrocin, which is characterized in that an endothelin core of erythrocin is prepared, and then an isolating layer, a protective layer, a second isolating layer and an improved enteric-coating material layer are applied one by one. In this way, new preparation of the erythrocin which has certain feature of releasing (dissolving) in acid solution (hydrochloric acid solution 9 to 1000) can be formed. The technology of the new preparation can also be widely applied to drugs which, like erythrocin, when being taken orally by a patient, cause the patient to suffer the side effects of stimulation, sickness and the like after degradation in the stomach of the patient or contact with the stomach of the patient, and drugs which the patient needs to take orally to let the blood concentration to reach the peak value in a short time. Such drugs include macrolides of azithromycin, metronidazole of nitroimidazoles, tinidazole, acyclovir as an antiviral drug, ammonium chloride as a phlegm eliminating drug, bromhexine, chloroquine as an antimalarial, nitroquine, artemisinin, dihydroartemisinin, artesunate, primaquine, pyrimethamine, carbarsone and emetine amebicides and so on.
Owner:胡昌勤 +1
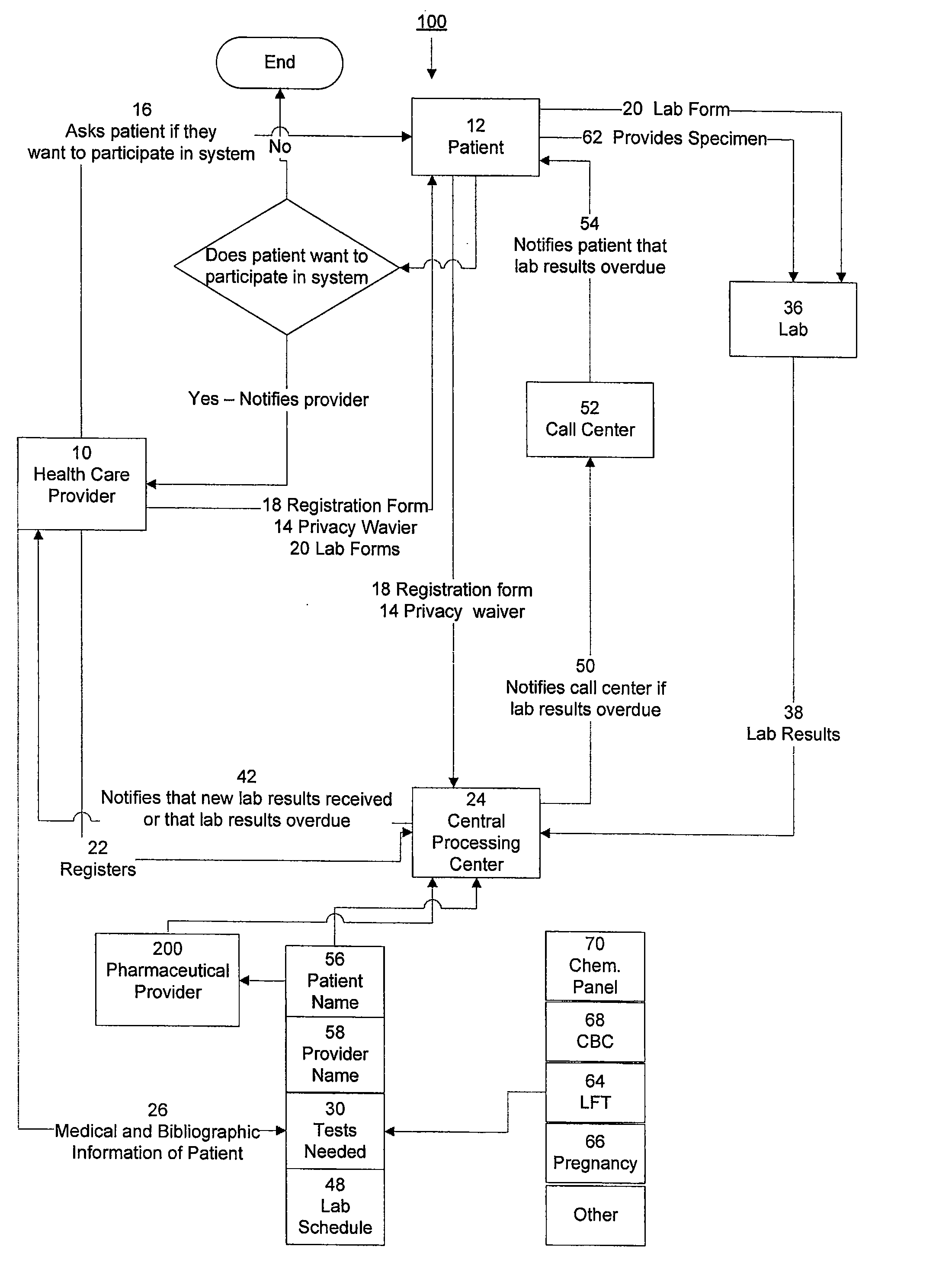
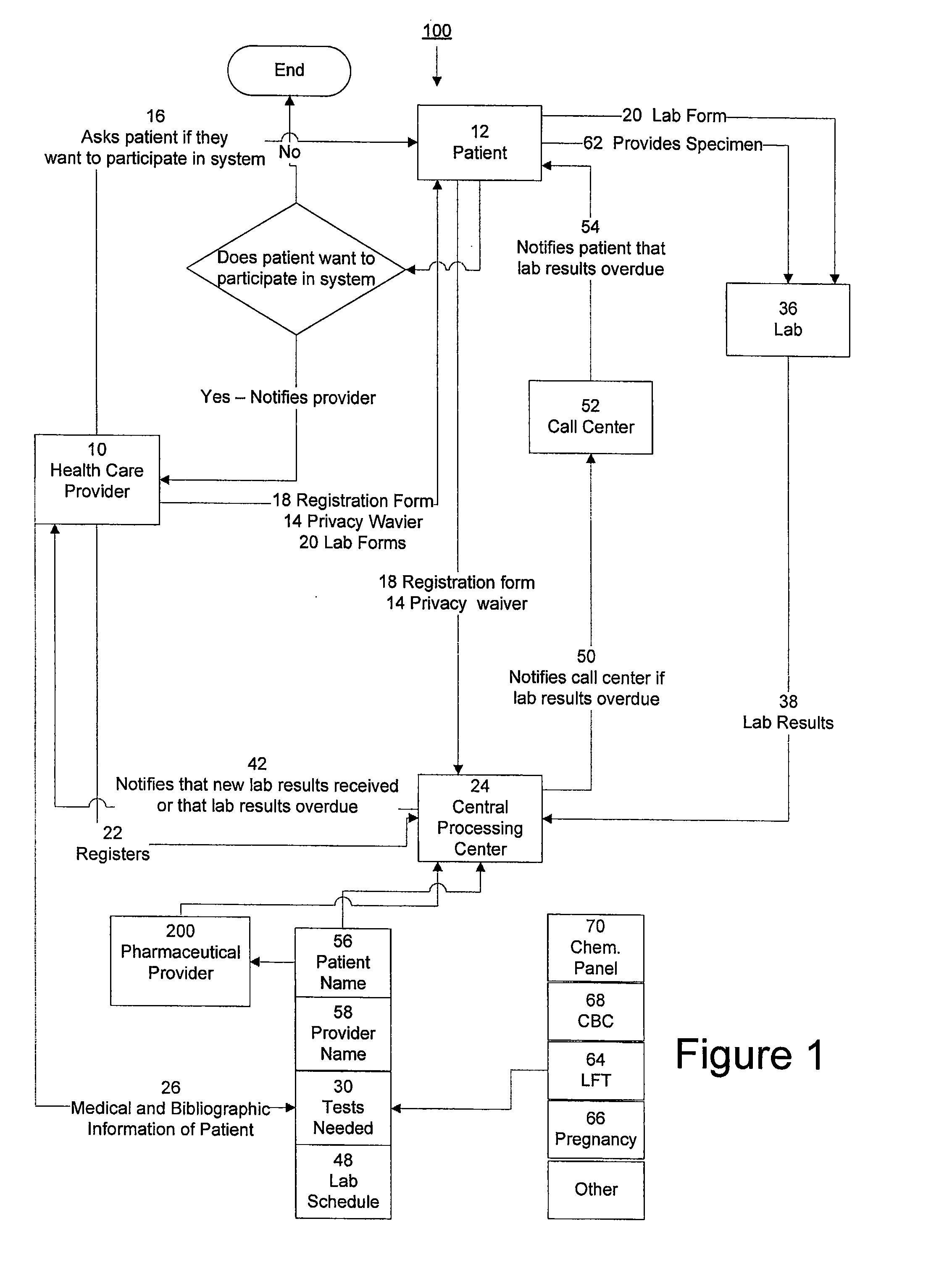
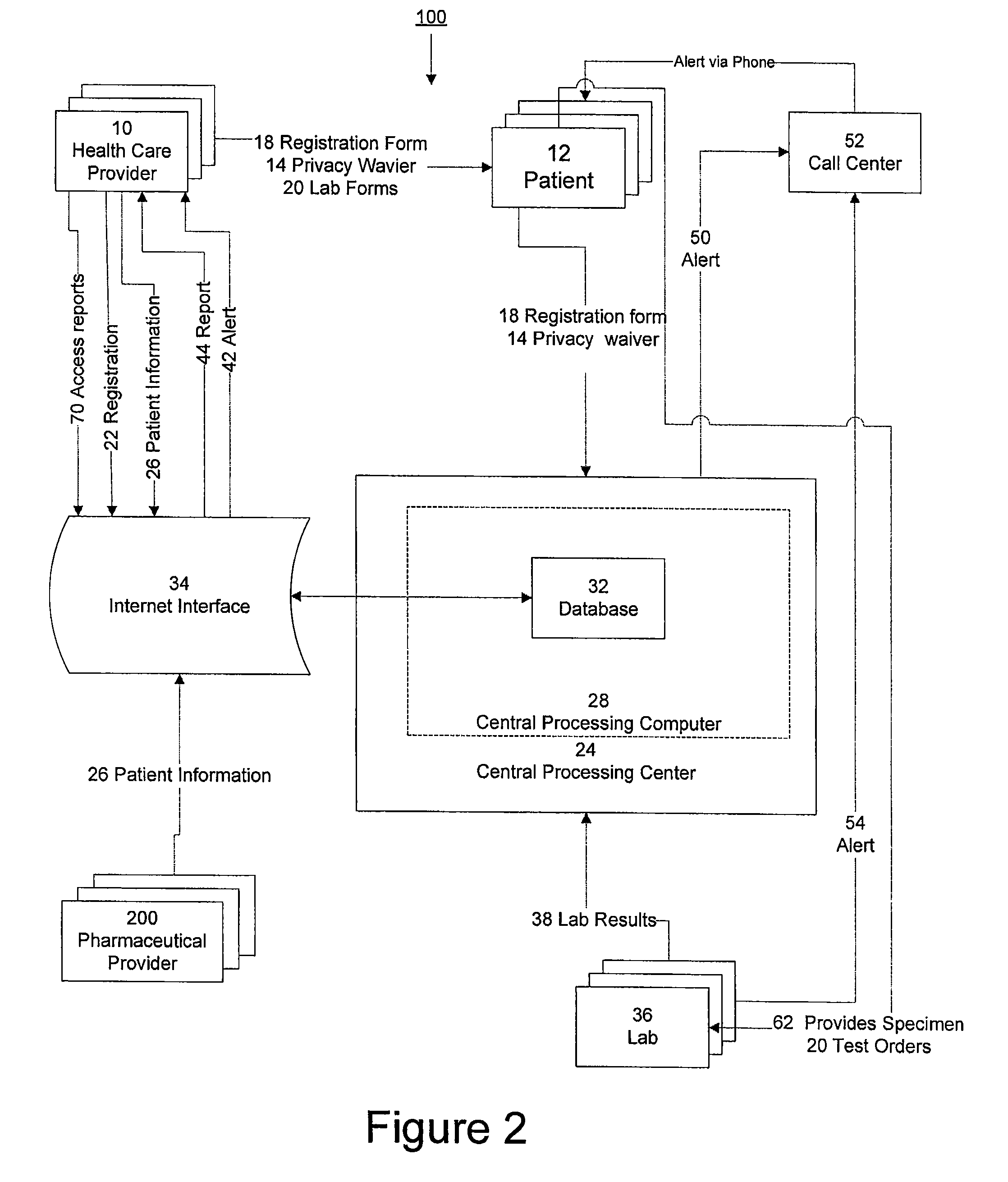
System for managing laboratory test results for patients taking an endothelin receptor antagonist
InactiveUS20090171694A1Computer-assisted medical data acquisitionResourcesTime scheduleLaboratory Test Result
The invention provides a system and method of collecting, storing and managing laboratory test results of patients. In one embodiment the invention is directed towards collecting, storing and managing laboratory test results of patients taking an endothelin receptor antagonist (ERA). The system provides a way of securely collecting and tracking information from a plurality of patients, a plurality of laboratories, and a plurality of health care providers by use of a database. The method comprises receiving patient information from health care providers, receiving a laboratory test schedule for each patient, and laboratory tests needed for each patient. This data is entered into a central processing computer. The patients then go to any laboratory to have the monthly testing done due to the ERA prescription. The laboratory test data collected is sent to the central processing computer. The central processing computer can generate an automated report on the laboratory results and supply the results to the health care provider. Additionally, if no laboratory test data for the patient is entered into the central processing computer in accordance with the laboratory test schedule for the patient, the central processing computer will notify a call center which will call the patient, laboratory or health care provider informing them of the overdue laboratory test data.
Owner:ACTELION PHARMA US
Pharmaceutical compositions comprising NEP-inhibitors, inhibitors of the endogenous endothelin producing system and AT1 receptor antagonists
InactiveUS20050288272A1Good curative effectImprove securityBiocideAnimal repellantsDepressantEssential hypertension
A combination therapy for cardiovascular diseases, in particular essential hypertension, pulmonary hypertension and / or congestive heart failure, involving administering a synergistic combination of at least one inhibitor of neutral endopeptidase, at least one inhibitor of the endogenous endothelin producing system, and at least one AT1 receptor antagonist.
Owner:SOLVAY PHARMA GMBH
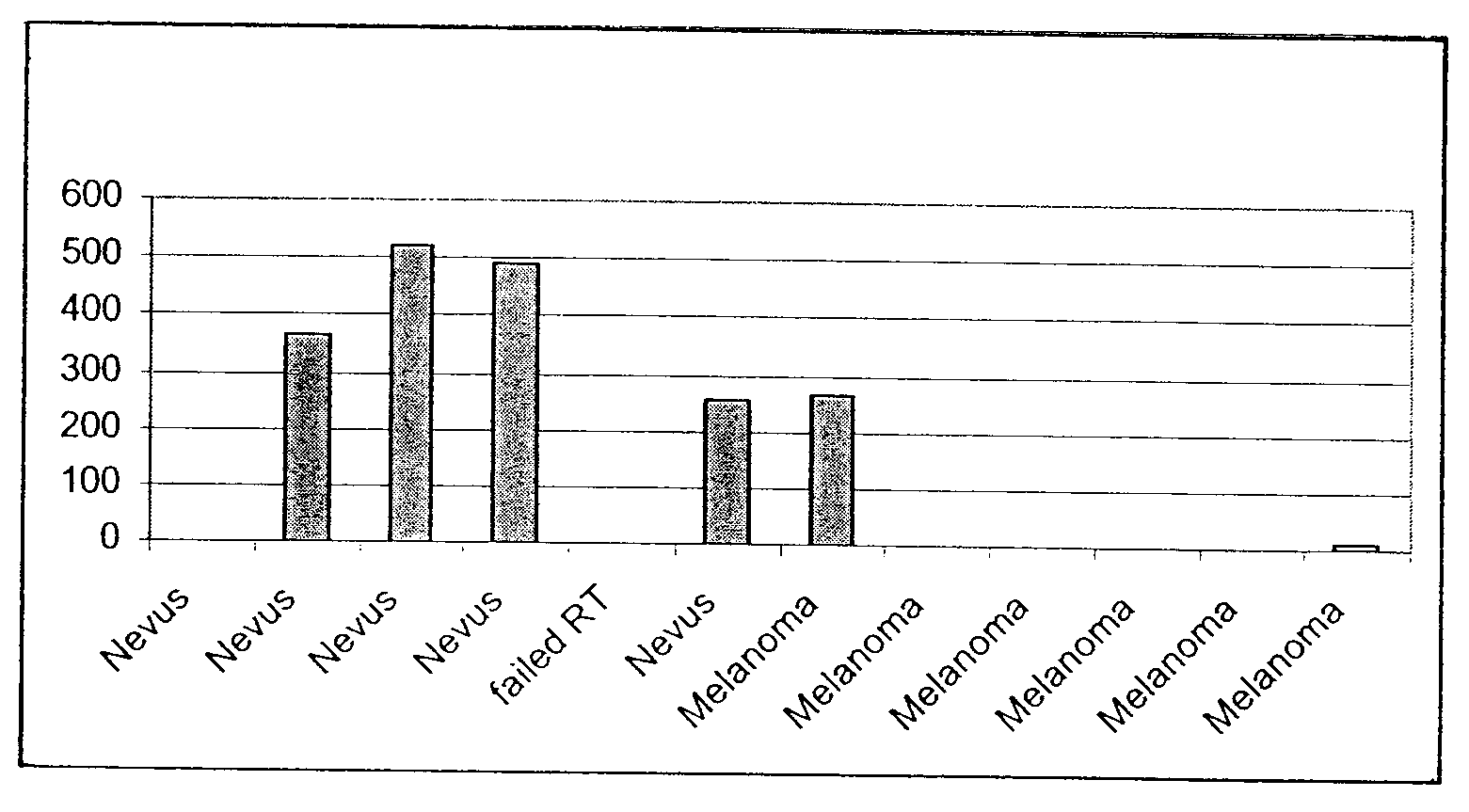
Method for Detection of Melanoma
The present invention provides non-invasive methods for detecting, monitoring, staging, and diagnosing malignant melanoma in a skin sample of a subject. The methods include analyzing expression in skin sample of one or more melanoma skin markers. The melanoma skin markers include IL-1 RI, endothelin-2, ephrin-A5, IGF Binding Protein 7, HLA-A0202 heavy chain, Activin A (βA subunit), TNF RII, SPC4, and CNTF Rα. The skin sample can include nucleic acids, and can be a human skin sample from a lesion suspected of being melanoma.
Owner:DERMTECH INT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com