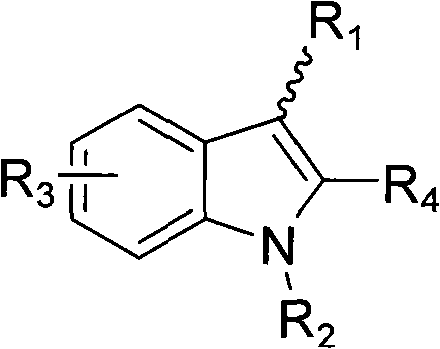
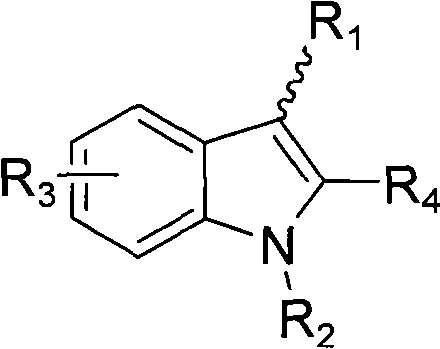
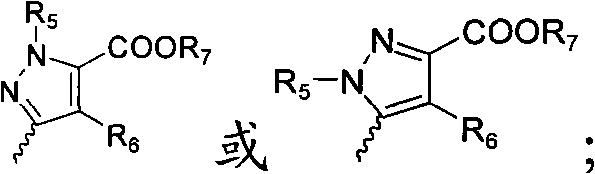
Indole ring substituted pyrazole carboxylic acid endothelin receptor antagonist as well as preparation method and application thereof
A technology of indole and pyrazole, which can be used in medical preparations containing active ingredients, pharmaceutical formulations, cardiovascular system diseases, etc., and can solve the problems of lack of treatment drugs and untimely treatment of pulmonary arterial hypertension.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0224] Example 1 Synthesis of Compound V
[0225]
[0226] Compound IV, indole (9.36 g, 80 mmol) was dissolved in anhydrous dichloromethane (160 ml), stirred under an ice-salt bath, and AlCl was added at -5-0 °C 3 (21.12 g, 160 mmol), pay attention to temperature control, slowly add acetic anhydride (7.5 ml, 80 mmol) dropwise at room temperature, add a small amount of nitromethane (30 ml), and react at room temperature for 5 hours. Ice water was added to the reaction solution, a large amount of solid was precipitated, filtered, dried, purified by column (chloroform / methanol, 20:1), and recrystallized from ethanol to obtain a pale yellow solid, namely compound V (3-acetylindole) 3.94g , the yield is 31%. mp 190-191℃, IR (KBr, cm -1 )3180, 1630, 1450, 1250, 1190, 945, 760; 1 H NMR (acetone-d 6 ,) δ8.7-7.0 (m, 5H), 2.4 (s, 3H).
Embodiment 2-8
[0228] Referring to the synthesis operation of Example 1, a different indole derivative was used to replace Compound IV in Example 1 to obtain a different 3-acetylindole derivative, Compound V.
[0229] Table 1 Compound V obtained in Example 2-Example 8
[0230]
Embodiment 9
[0231] Example 9 Synthesis of Compound IV
[0232]
[0233] Compound V, 3-acetyl indole (2.1 g, 13.2 mmol) and diethyl oxalate (2.51 g, 17.2 mmol) were mixed, stirred, added with anhydrous sodium ethoxide (1.8 g, 26.4 mmol), nitrogen protection, room temperature reaction 5 hours. Dilute acetic acid was added to the reaction solution to produce a large amount of solid, suction filtration, the filter cake was washed three times with diluted acetic acid, and the filter cake was dried to obtain a yellow solid, compound IV, 3.4g, yield 100%, melting point: 180.8-182.7°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com