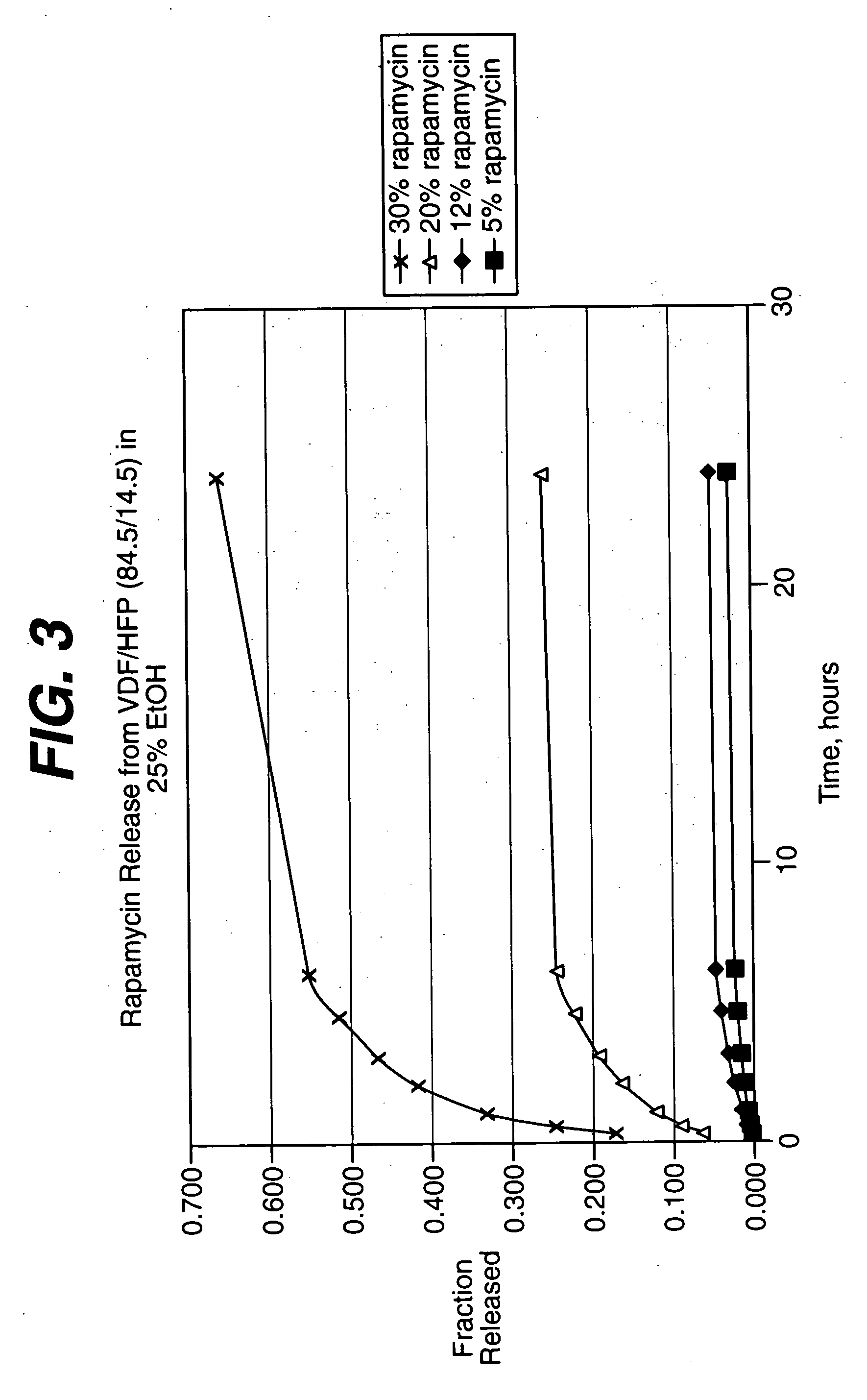
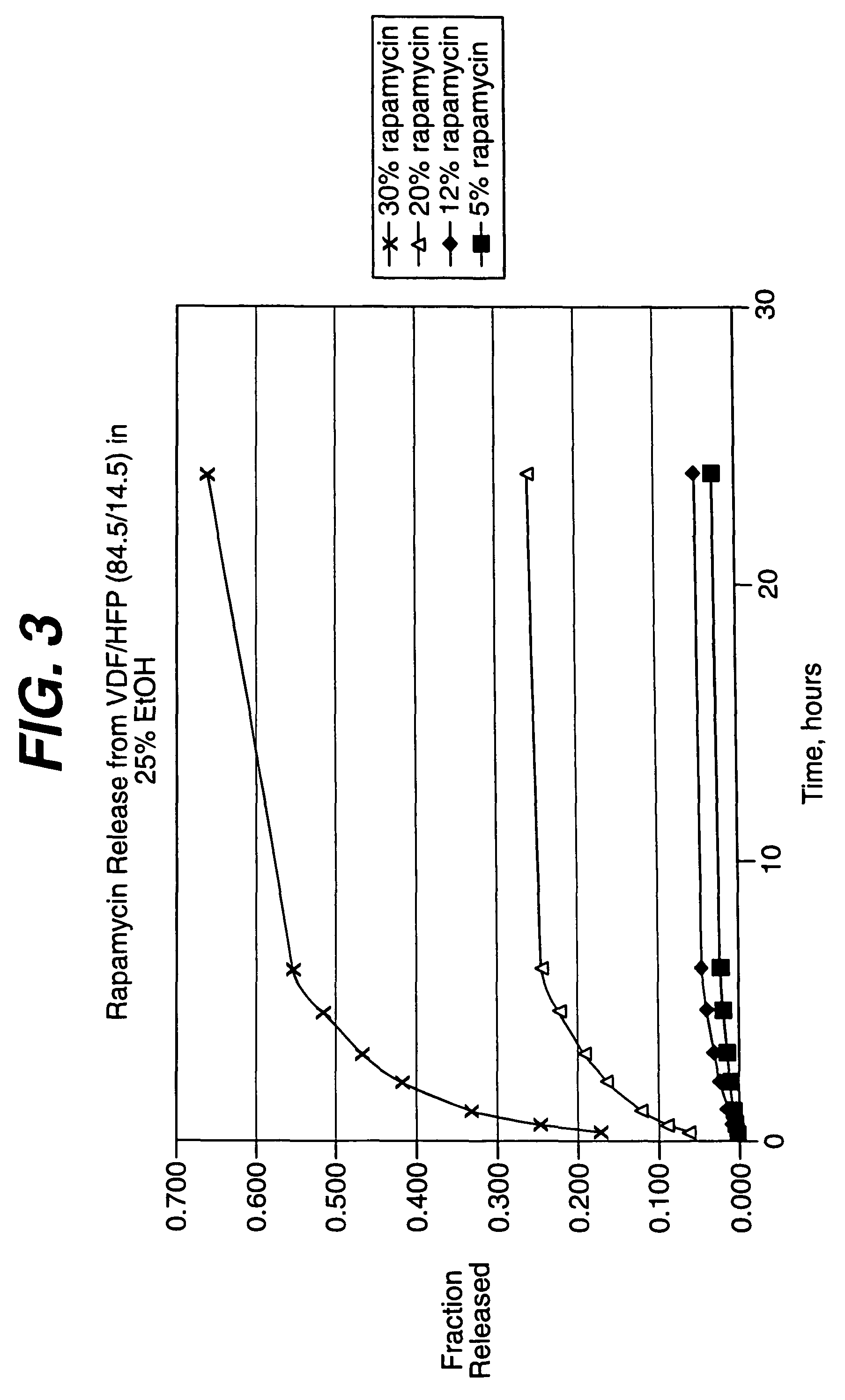
Patents
Literature
289 results about "Organic body" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
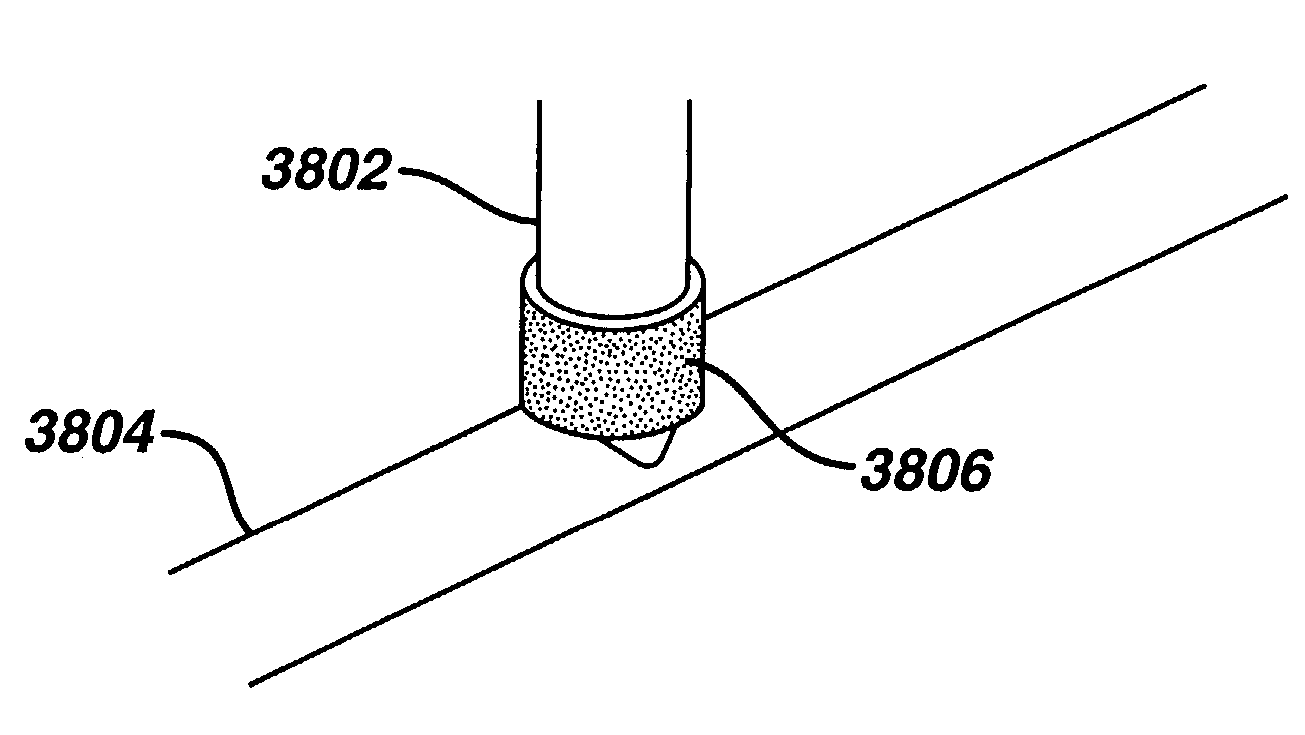
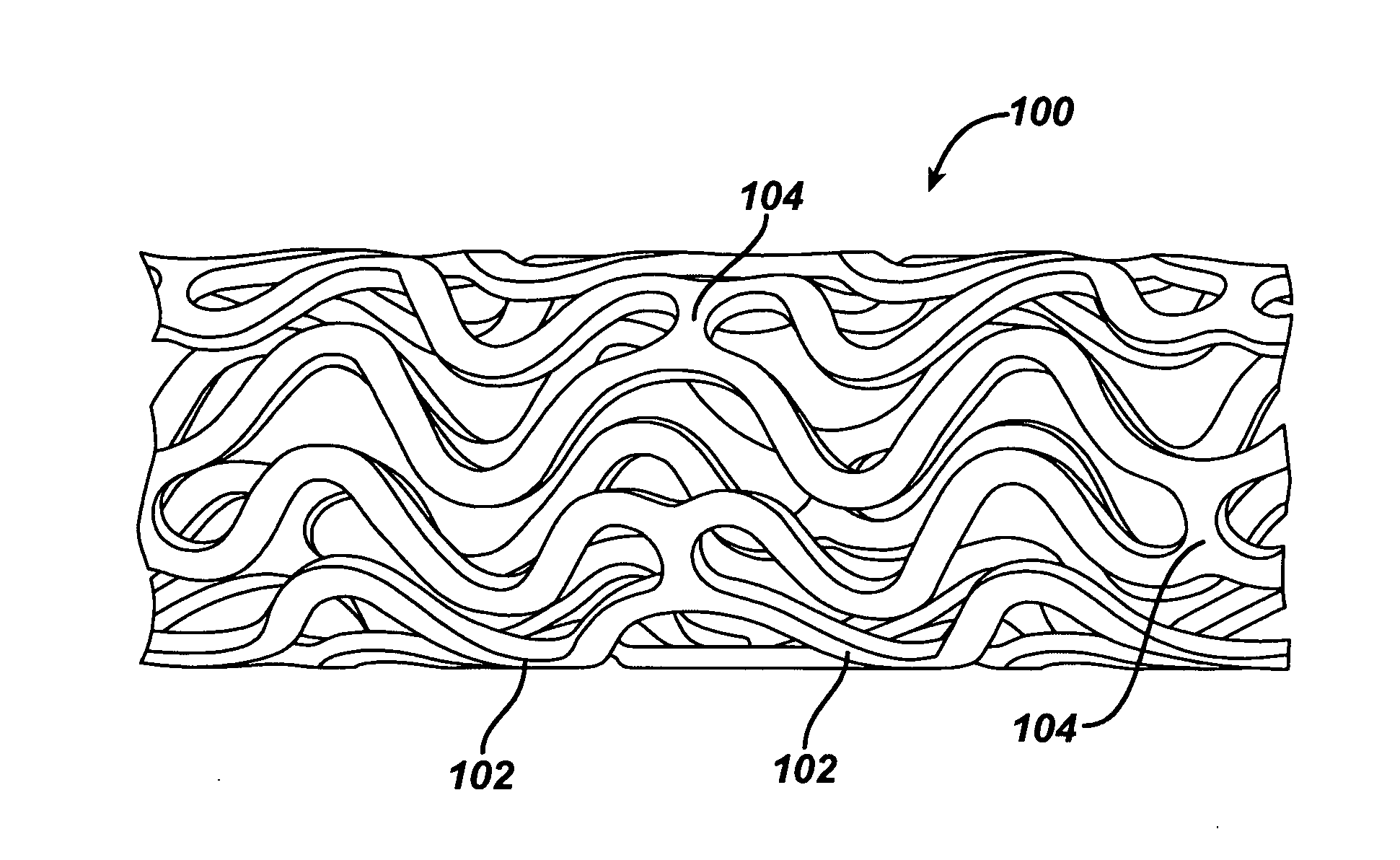
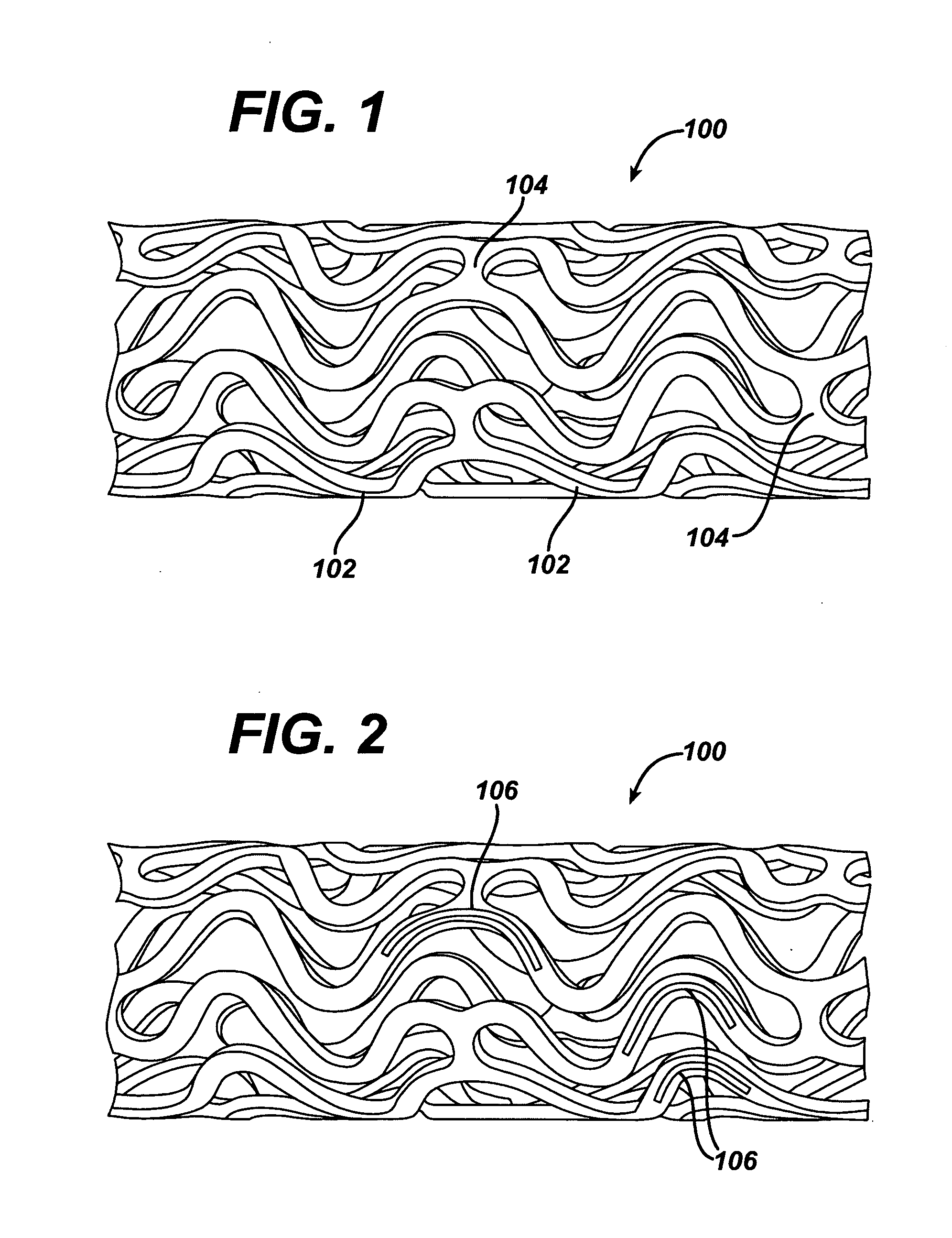
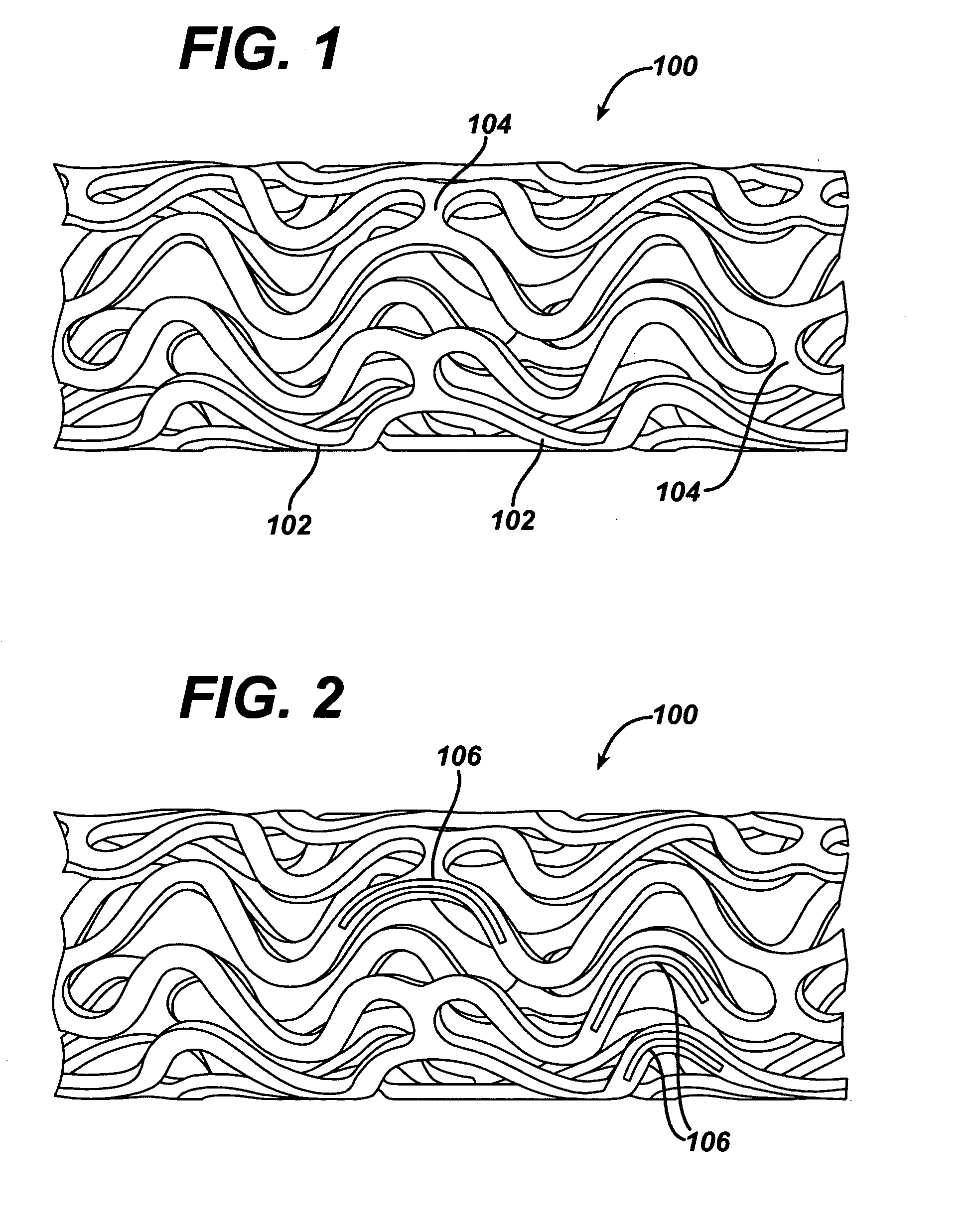
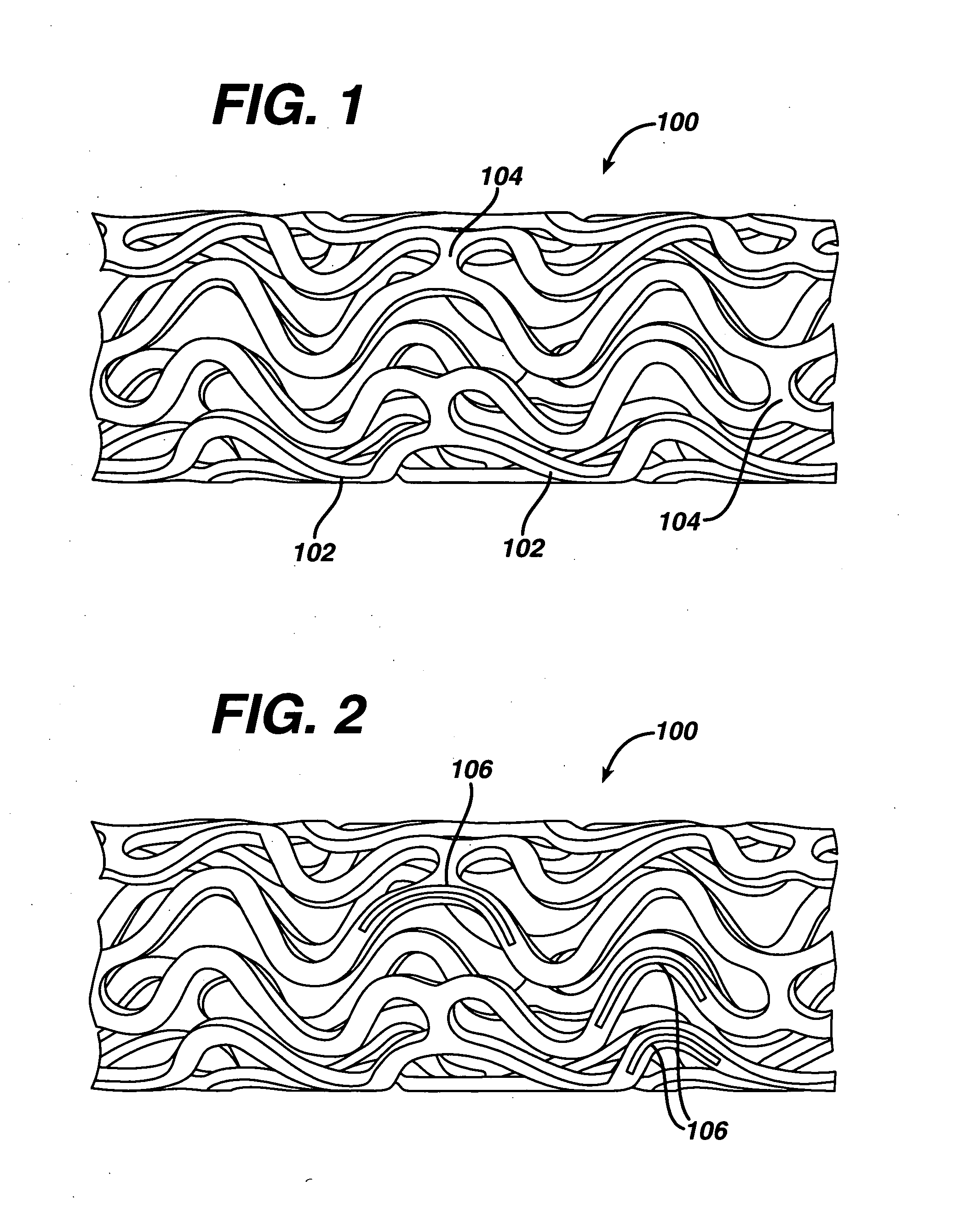
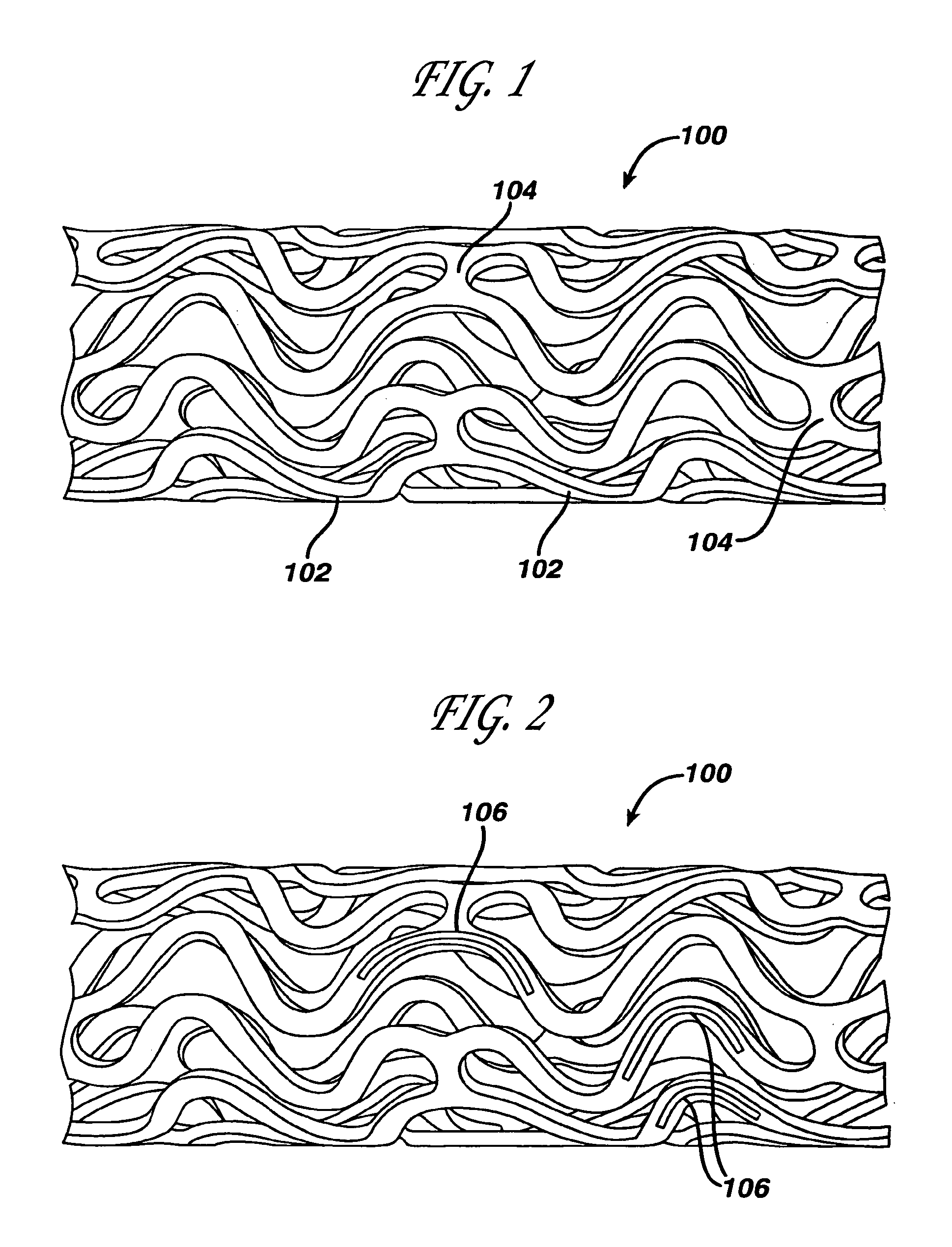
Drug releasing anastomosis devices and methods for treating anastomotic sites
ActiveUS7108701B2Reduce drug toxicityGood curative effectSuture equipmentsSurgical needlesBiological bodyReady to use
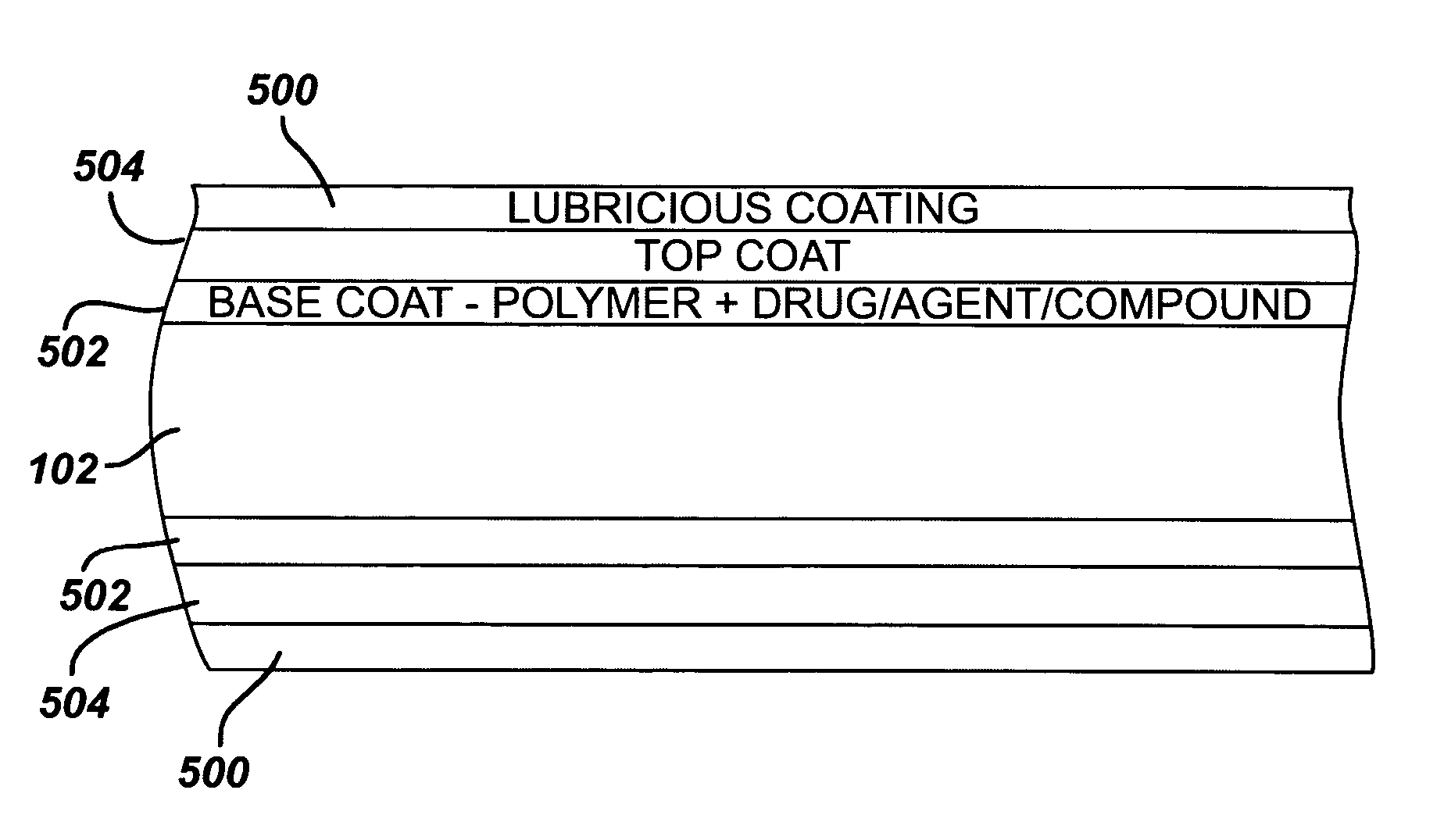
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:WYETH
Coated endovascular AAA device
InactiveUS6852122B2Reduce drug toxicityGood curative effectSuture equipmentsStentsBiological bodyReady to use
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Modified delivery device for coated medical devices
InactiveUS7527632B2Minimize potential risk of damageReduce frictionStentsEar treatmentBiological bodyMedical device
Medical devices, and in particular implantable medical devices, including self-expanding stents, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Endovascular graft with differentiable porosity along its length
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device such as a stent-graft. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. A stent-graft fabricated from a thin-walled, high strength material provides for a more durable and lower profile endoprosthesis. The stent-graft comprises one or more stent segments covered with a fabric formed by the weaving, knitting or braiding of a biocompatible, high tensile strength, abrasion resistant, highly durable yarn such as ultra high molecular weight polyethylene. The one or more stent segments may be balloon expandable or self-expanding. The fabric may be attached to the stent segments utilizing any number of known materials and techniques. In addition, the pore size of the graft material may be varied.
Owner:CORDIS CORP
Coating for controlled release of a therapeutic agent
InactiveUS20050033417A1Minimize potential risk of damageReduce frictionSuture equipmentsOrganic active ingredientsBlood vesselPolymer
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH
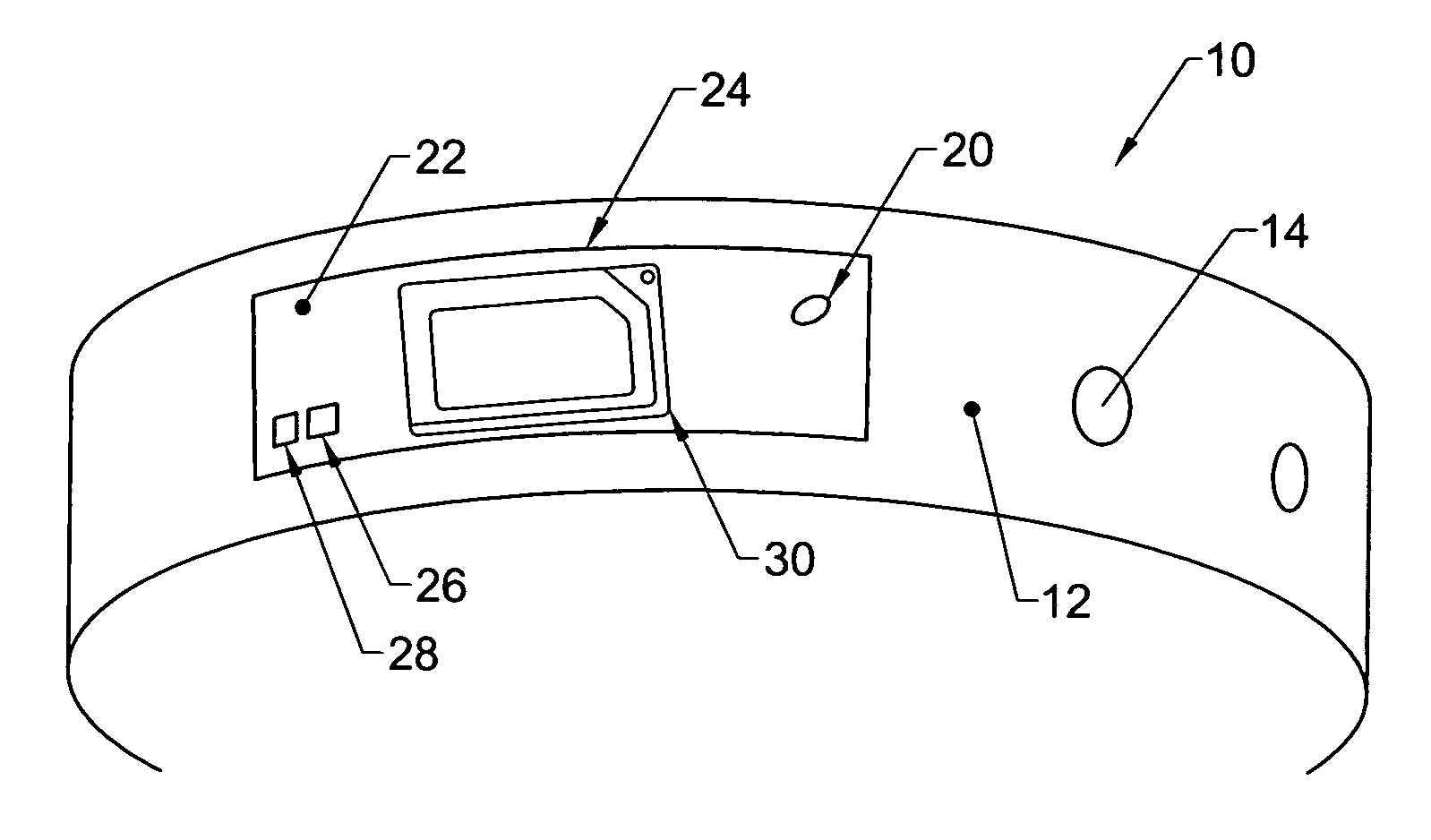
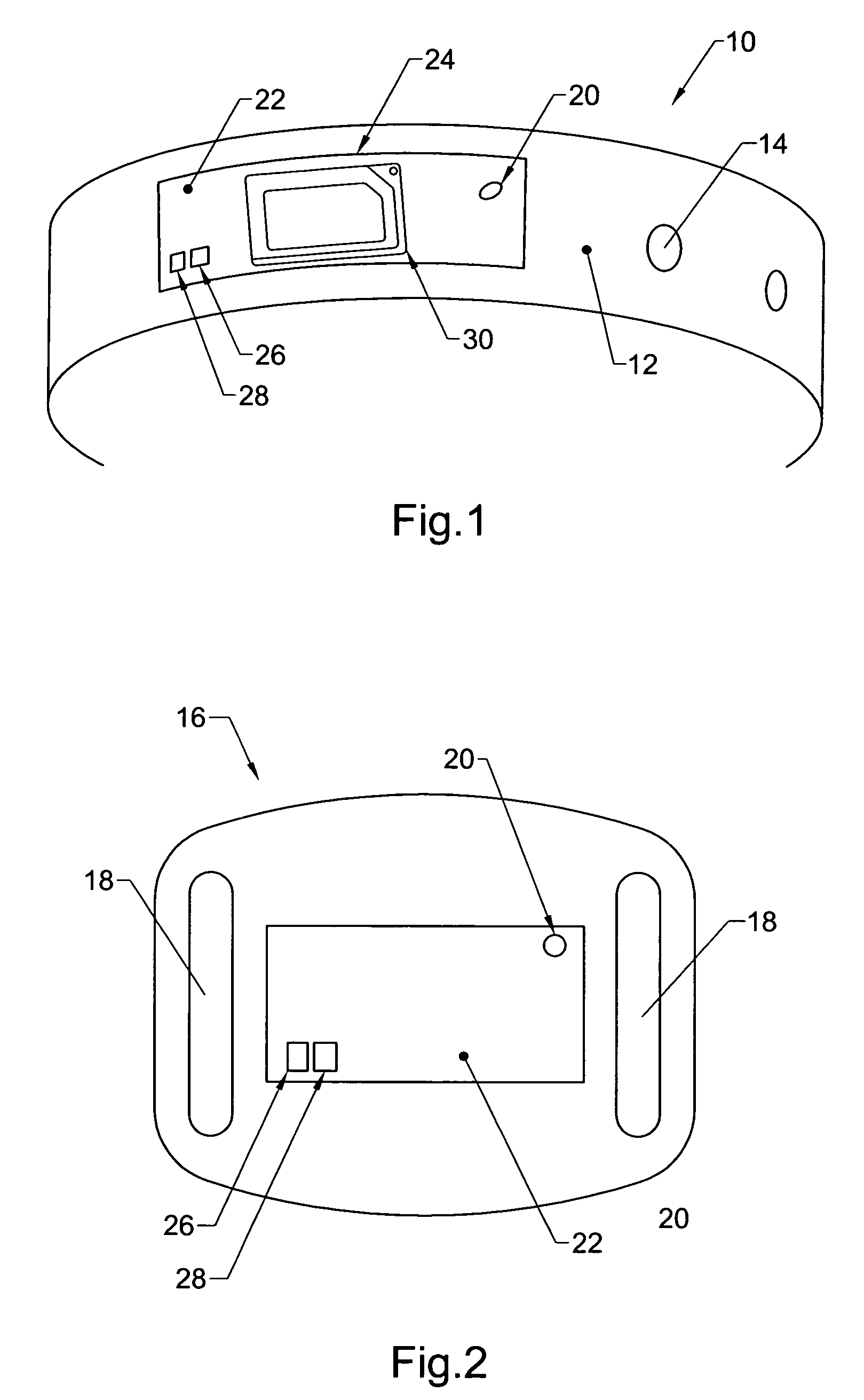
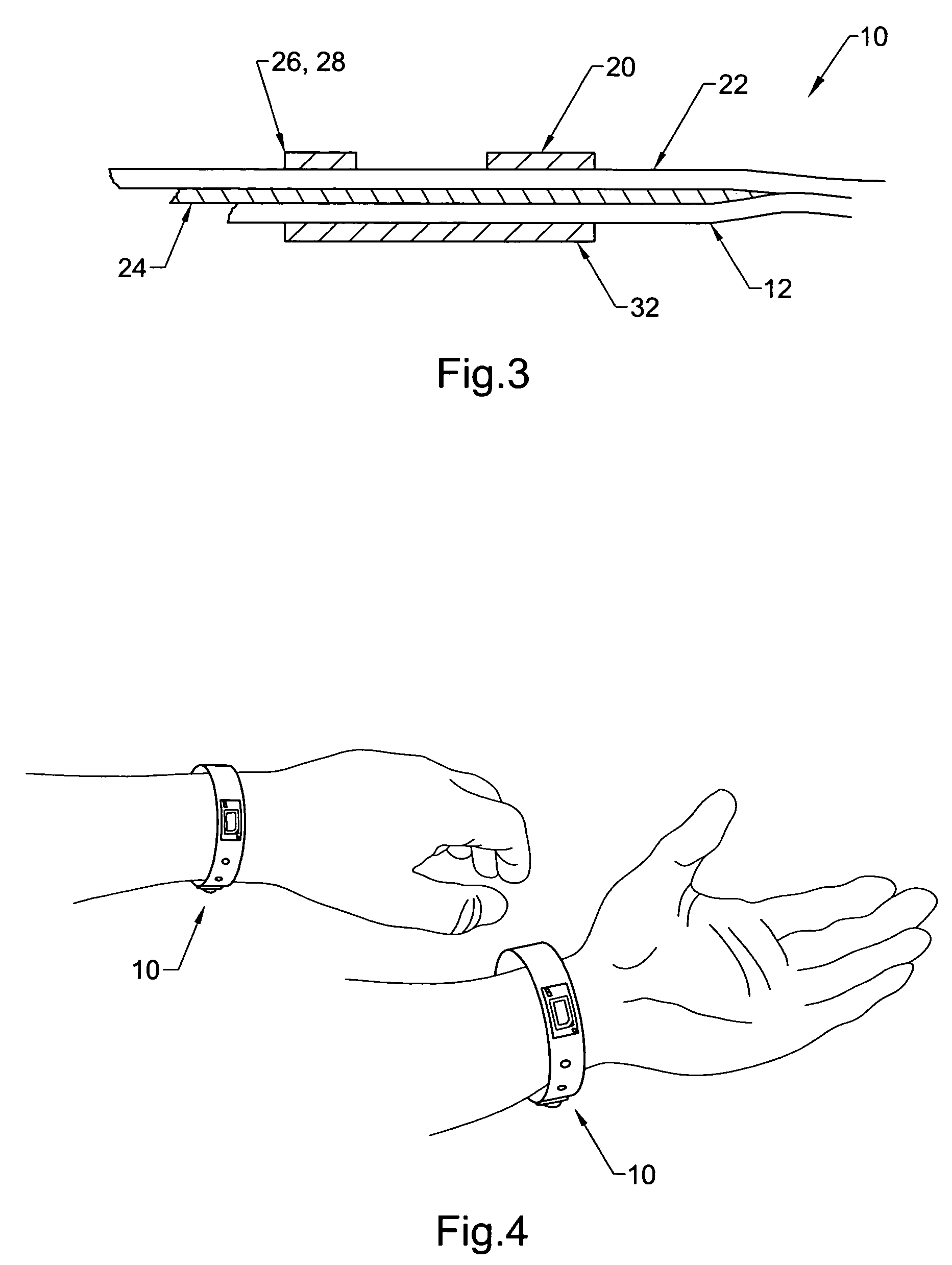
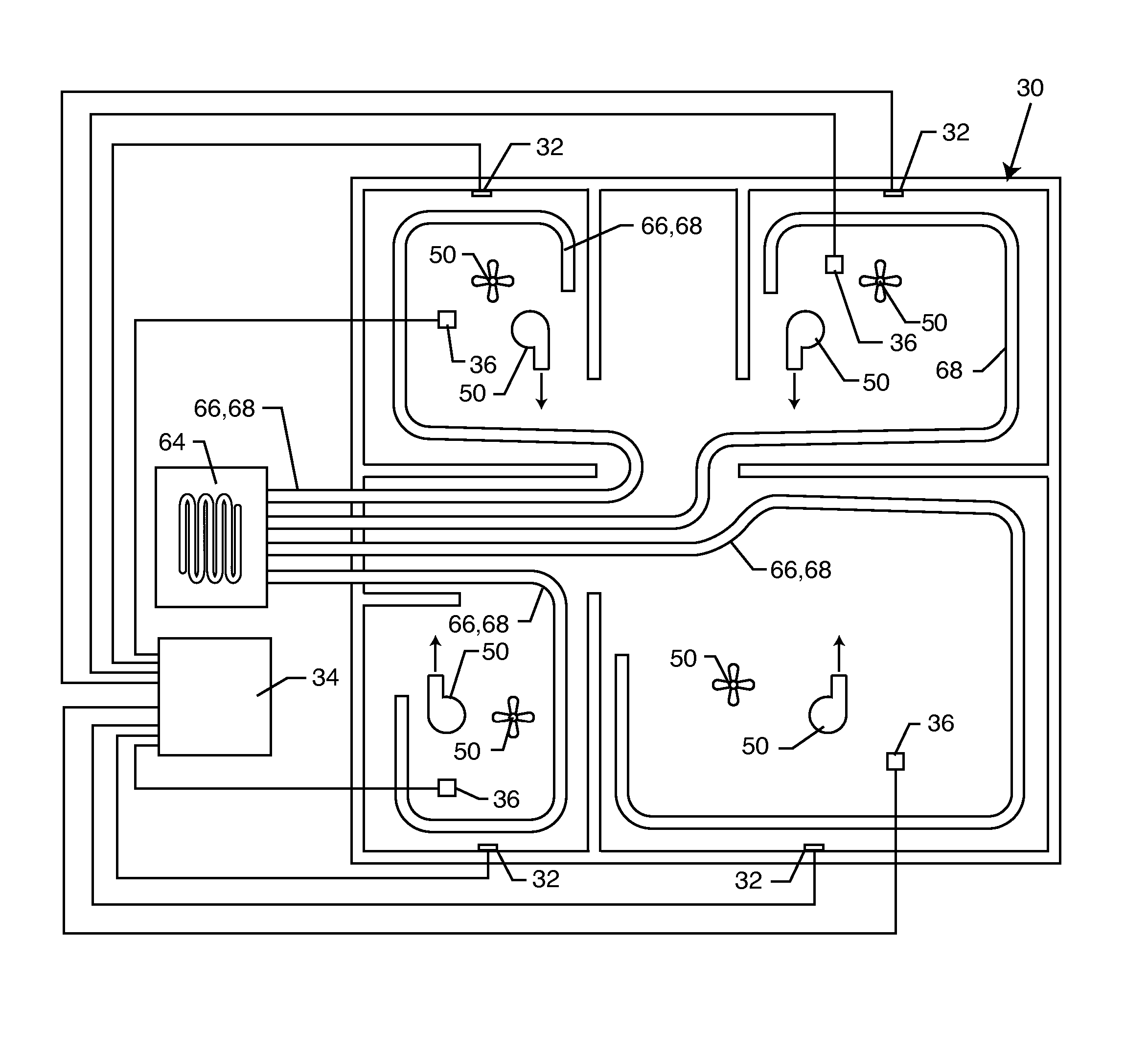
Physical condition or environmental threat detection appliance system
InactiveUS7188767B2Converting sensor ouput using wave/particle radiationMeasurement arrangements for variableEngineeringRadio frequency
A system includes one or more devices for providing information about a physical condition or environmental hazard. Each device includes a sensor physically associated with the user for detecting a predetermined environmental hazard and / or a physical condition of the user. The device is usually in the form of a wristband patch or tag attached to the user. The one or more sensors are adapted to detect predetermined chemicals, biological organisms, radiation or user blood pressure, heart rate, temperature, oxygen level, glucose level, skin condition, or biological and chemical changes of the user. An electronic circuit communicates with the sensor and includes an alarm for notifying the user or a third party of elevated environmental hazards or abnormal physical conditions of the user. Typically, the electronic circuit includes a transmitter, such as a radio frequency transmitter, for conveying information obtained from the sensor and electronic circuitry to a third party.
Owner:PRECISION DYNAMICS CORPORATION
Remote physiological monitoring
A system for remote physiological monitoring comprises a communication device and a patch. The patch is configured to be removably securable to a body of a biological organism and further configured to monitor a physiological parameter of a biological organism. The communication device is configured to receive communications from the patch. The communication device is further configured to transmit communications to a remote location.
Owner:AVERY DENNISON CORP
Coated medical devices for the treatment of vulnerable plaque
InactiveUS7195640B2Reduce drug toxicityGood curative effectSuture equipmentsSurgical needlesPercent Diameter StenosisCoating
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. In addition to reducing or substantially eliminating a biological organism's reaction to the introduction of the medical device to the organism, the medical device in combination with one or more therapeutic drugs, agents and / or compounds may be utilized to treat various vascular diseases, for example, restenosis and vulnerable plaque. In the case of vulnerable plaque, one or more drugs, agents or compounds may be utilized to treat the various aspects of vulnerable plaque and these drugs, agents and / or compounds may be released with a given release profile for the most effective treatment. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH +1
Injectable formulations of taxanes for cad treatment
InactiveUS20050272806A1Minimize potential risk of damageReduce frictionBiocideOrganic active ingredientsAntioxidantBlood vessel
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices. Liquid formulations, including solutions and suspensions of the various drugs, agents and / or compounds, may be locally or regionally delivered. In each of these instances, antioxidants are utilized to prolong product integrity.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Intraluminal medical devices in combination with therapeutic agents
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH
Thin-film nitinol based drug eluting stent
InactiveUS20070173787A1Reduce drug toxicityGood curative effectStentsPharmaceutical delivery mechanismDiseaseBlood vessel
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. These devices may also comprise thin films that perform a number of functions. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:CORDIS CORP
Medical devices, drug coatings and methods for maintaining the drug coatings thereon
InactiveUS7056550B2Reduce drug toxicityGood curative effectSuture equipmentsOrganic active ingredientsBiological bodyMedical device
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:WYETH LLC +1
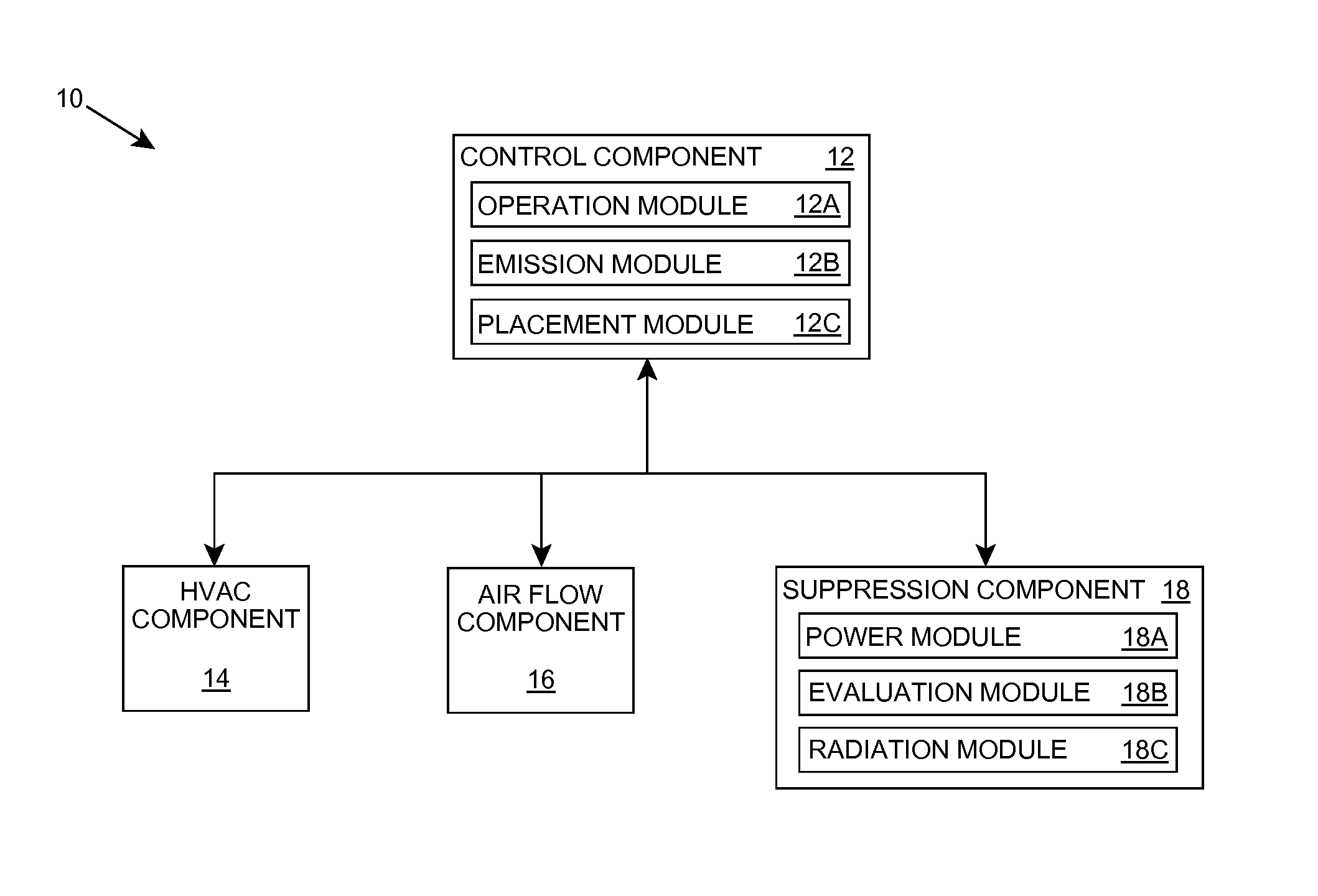
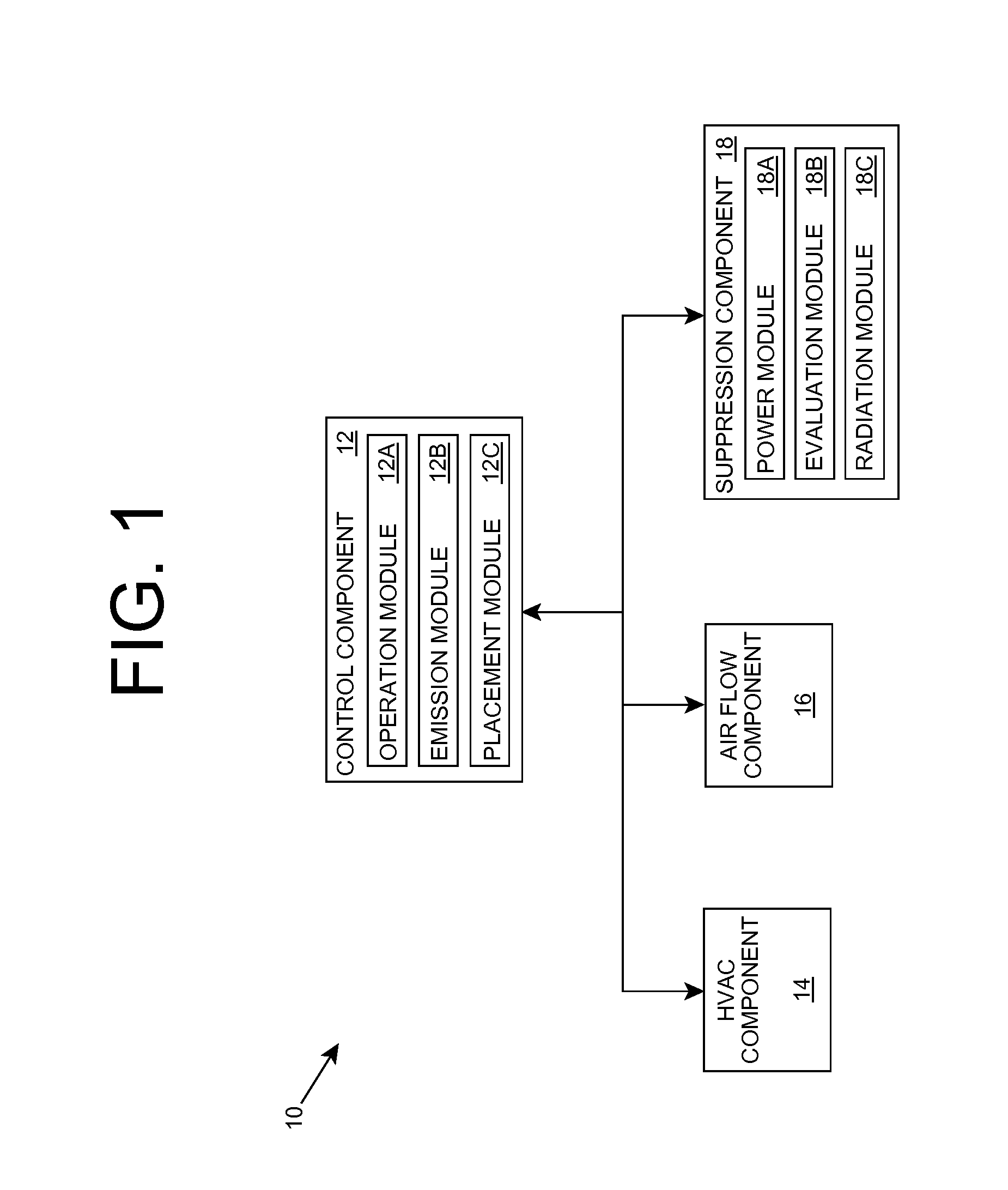
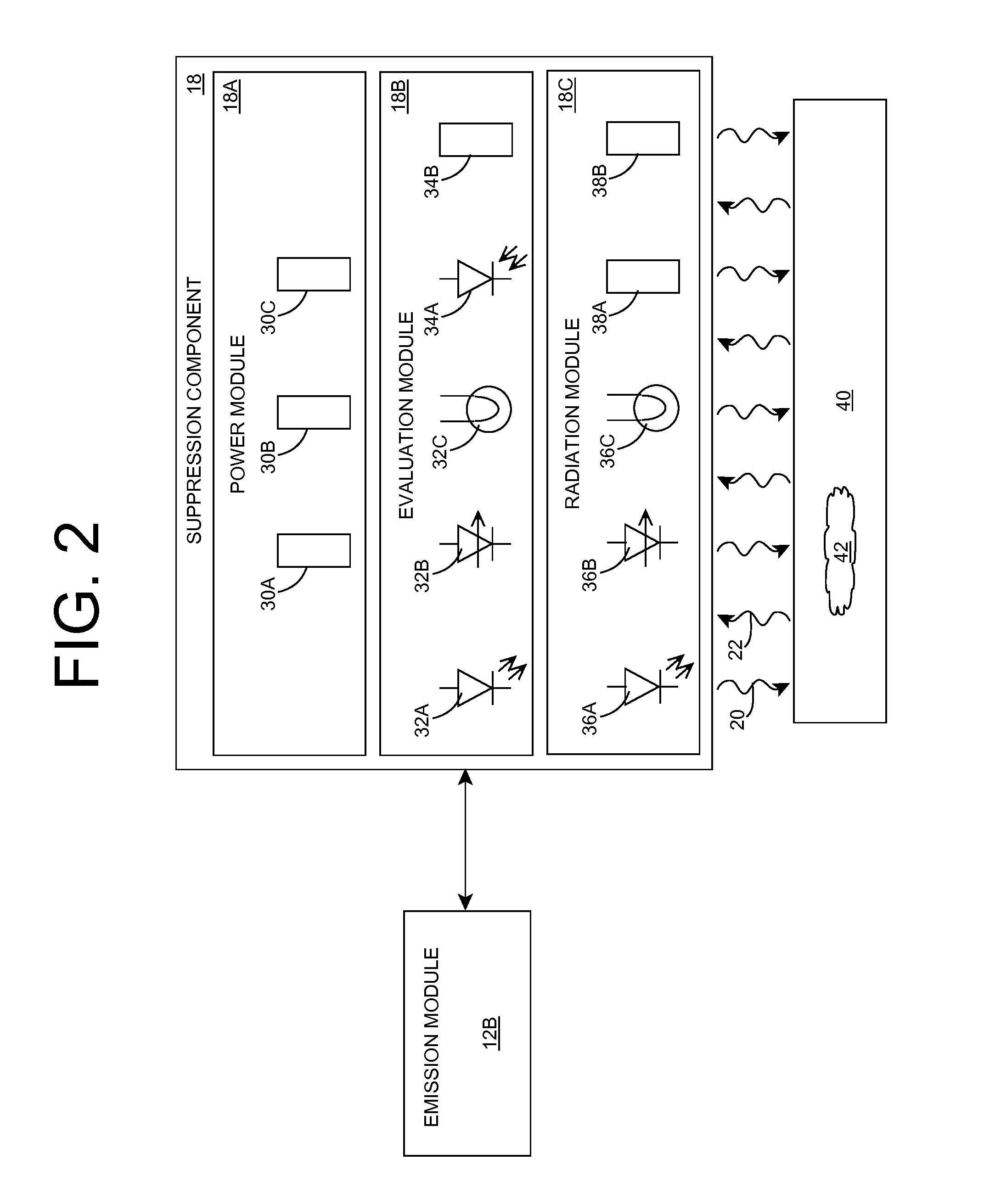
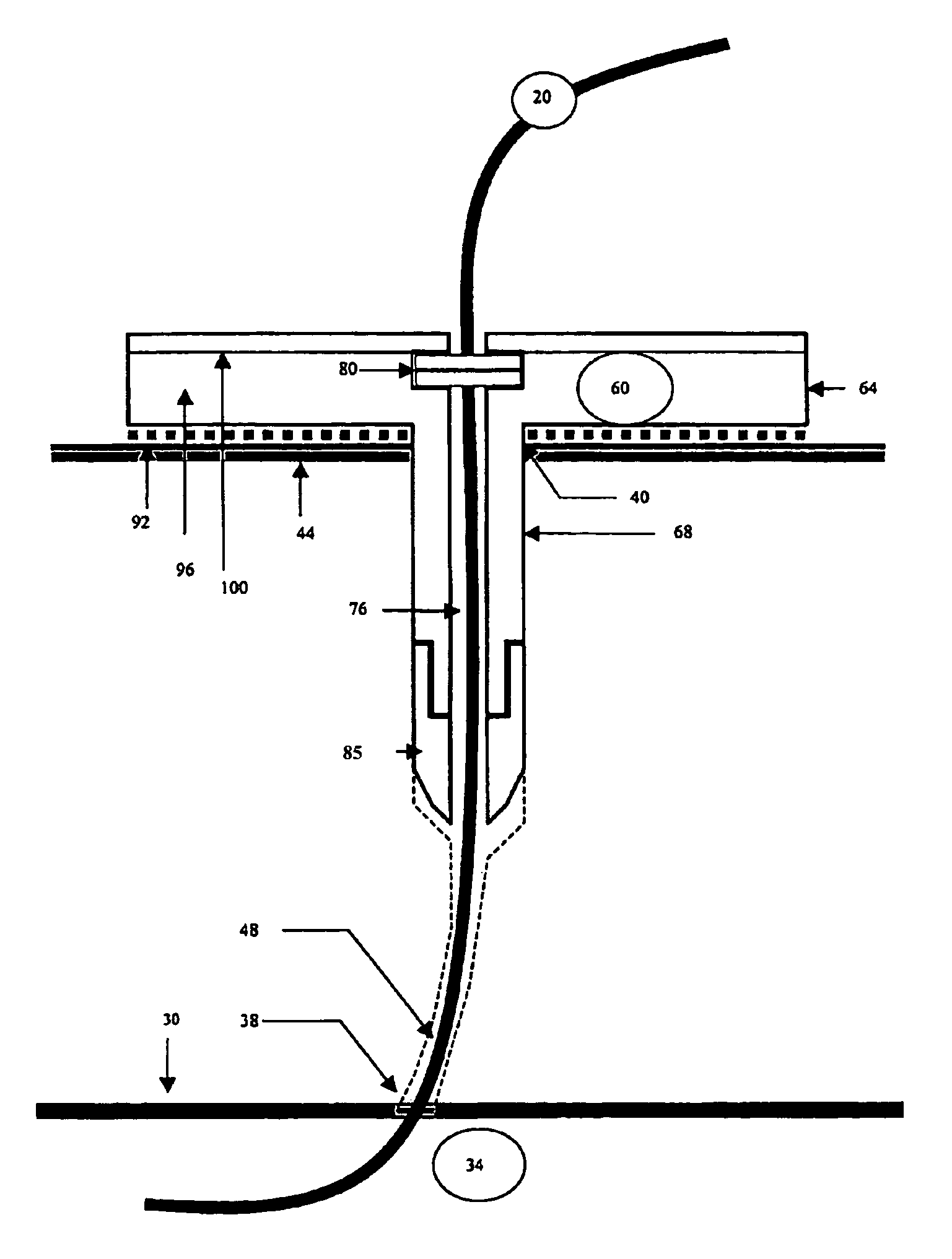
Organism growth suppression using ultraviolet radiation
ActiveUS20070205382A1Suppress and prevent growthGrowth inhibitionRadiation pyrometryFire rescueBiological bodyBiofilm
A solution for suppressing organism growth using ultraviolet radiation generated by solid state ultraviolet radiation emitters, such as ultraviolet diodes is provided. The invention includes a connection structure that includes a plurality of solid state ultraviolet radiation emitters disposed thereon. Each of the plurality of solid state ultraviolet radiation emitters emits ultraviolet radiation having a wavelength less than or equal to four hundred nanometers to harm a target organism that may be present on a surface. In one embodiment, the connection structure comprises a two-dimensional mesh that may be placed adjacent an air filter, incorporated in a cover, and / or moved with respect to a surface, such as the interior of an air duct. In this manner, the invention can suppress and / or prevent the growth of organisms, such as biofilms and mold, in locations that are susceptible to such growth.
Owner:SENSOR ELECTRONICS TECH
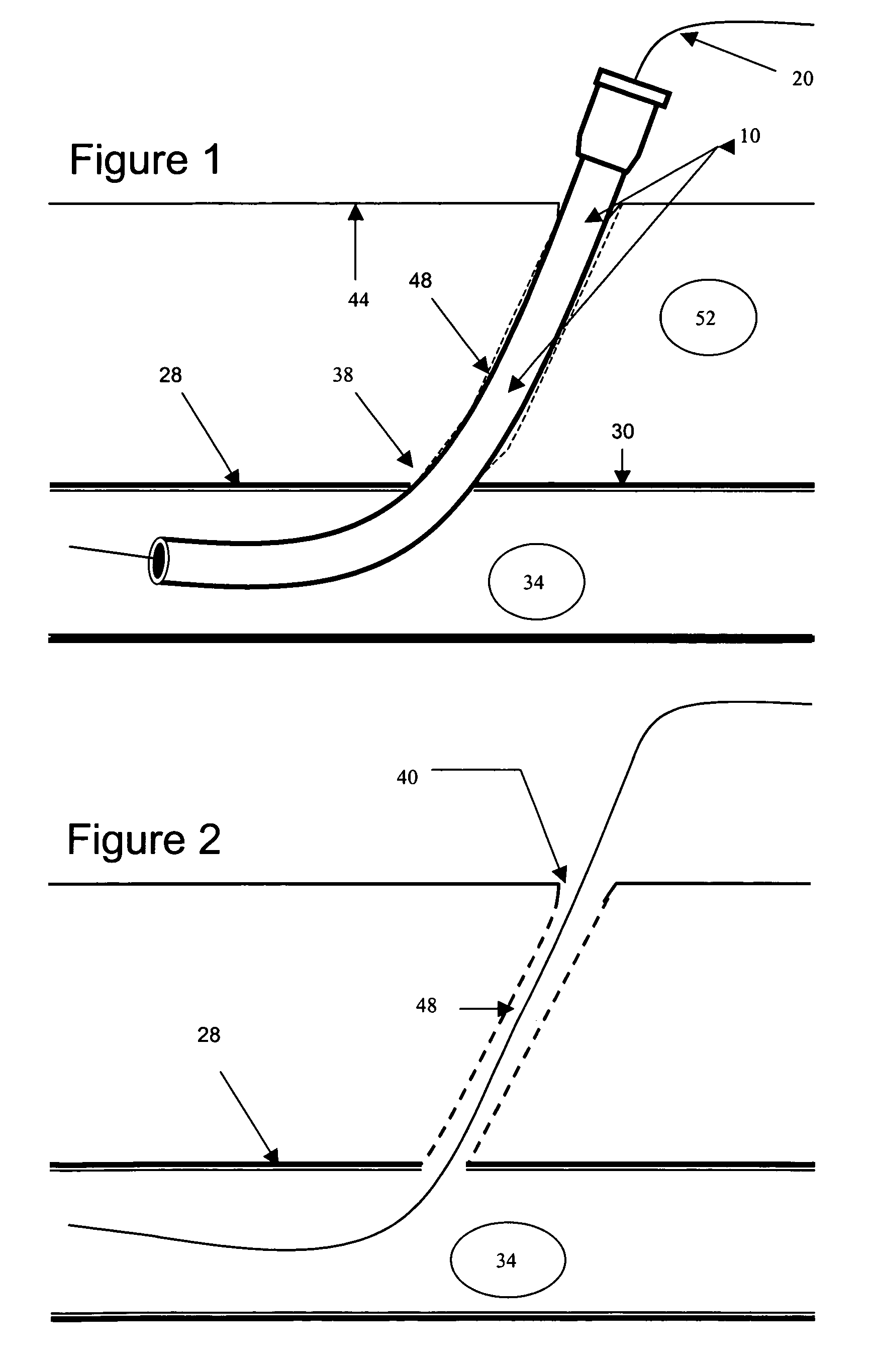
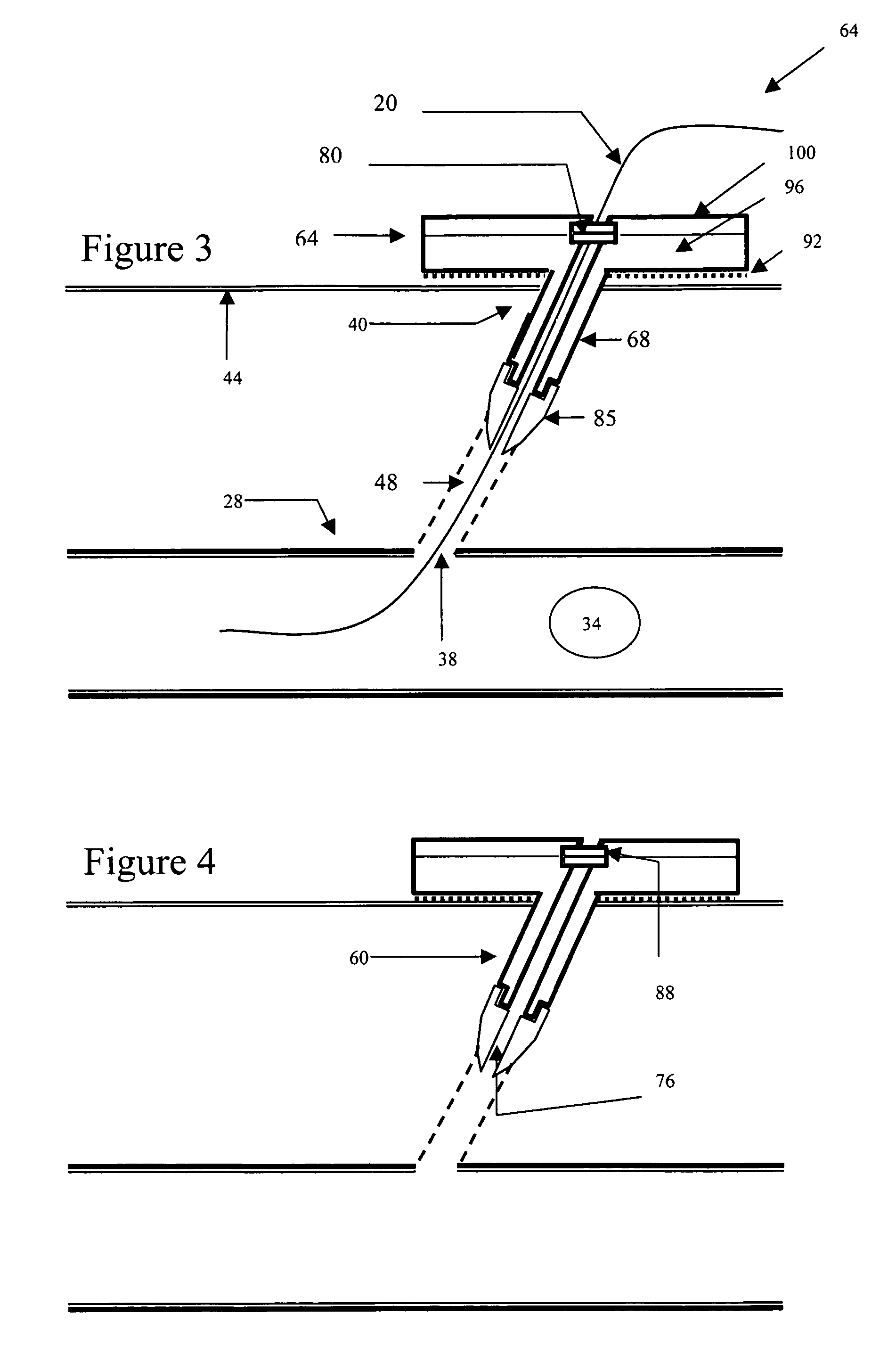
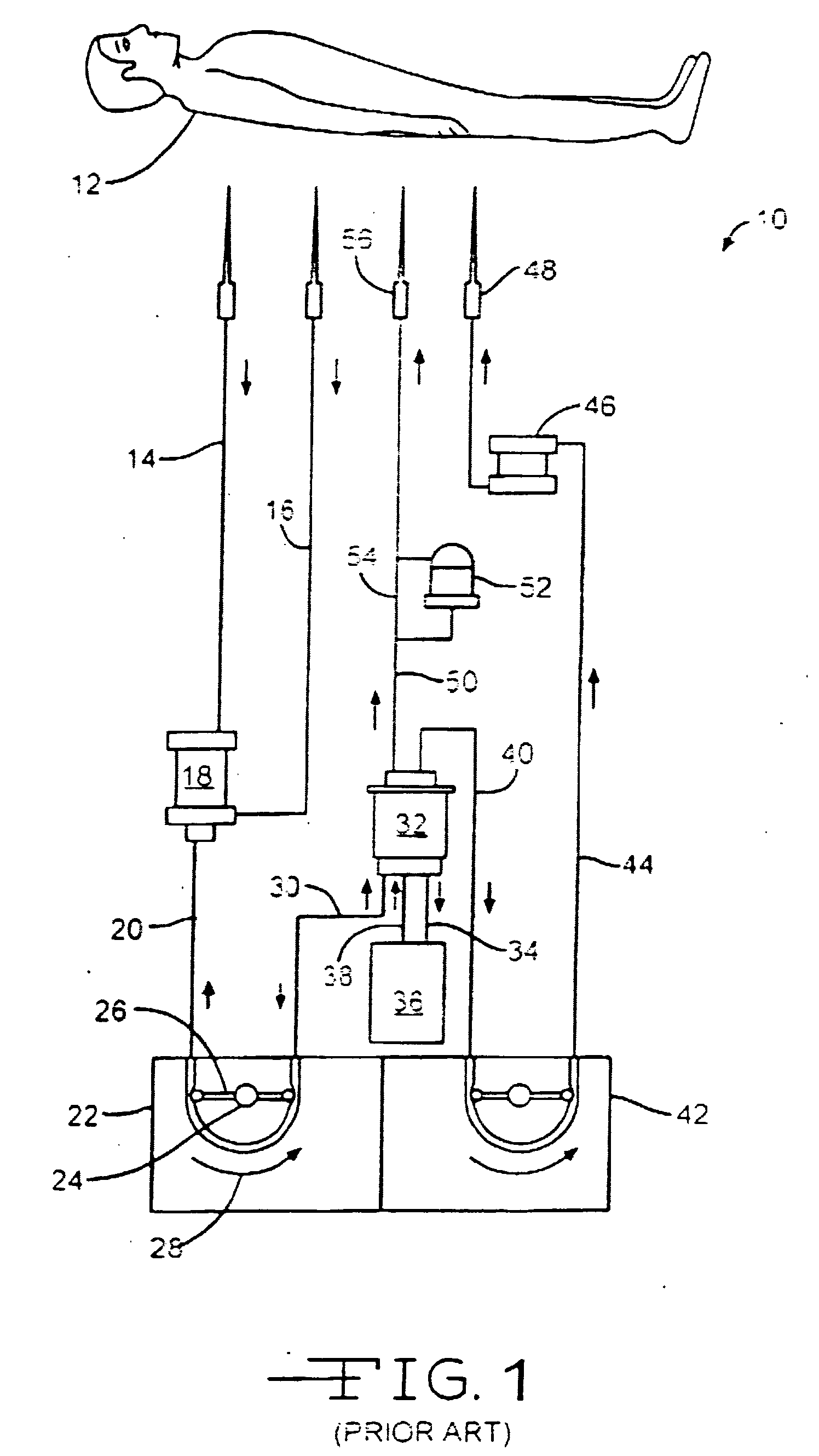
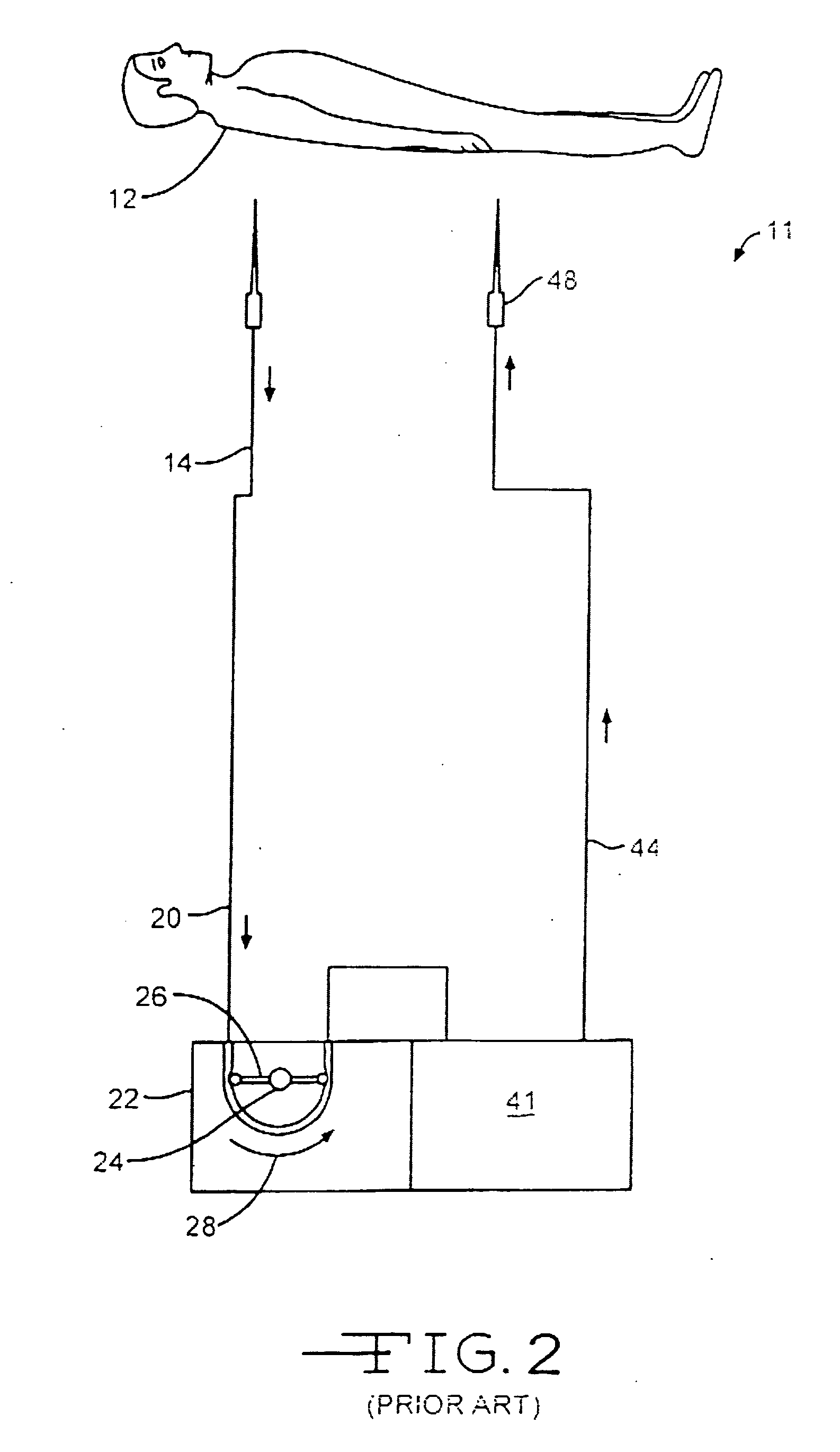
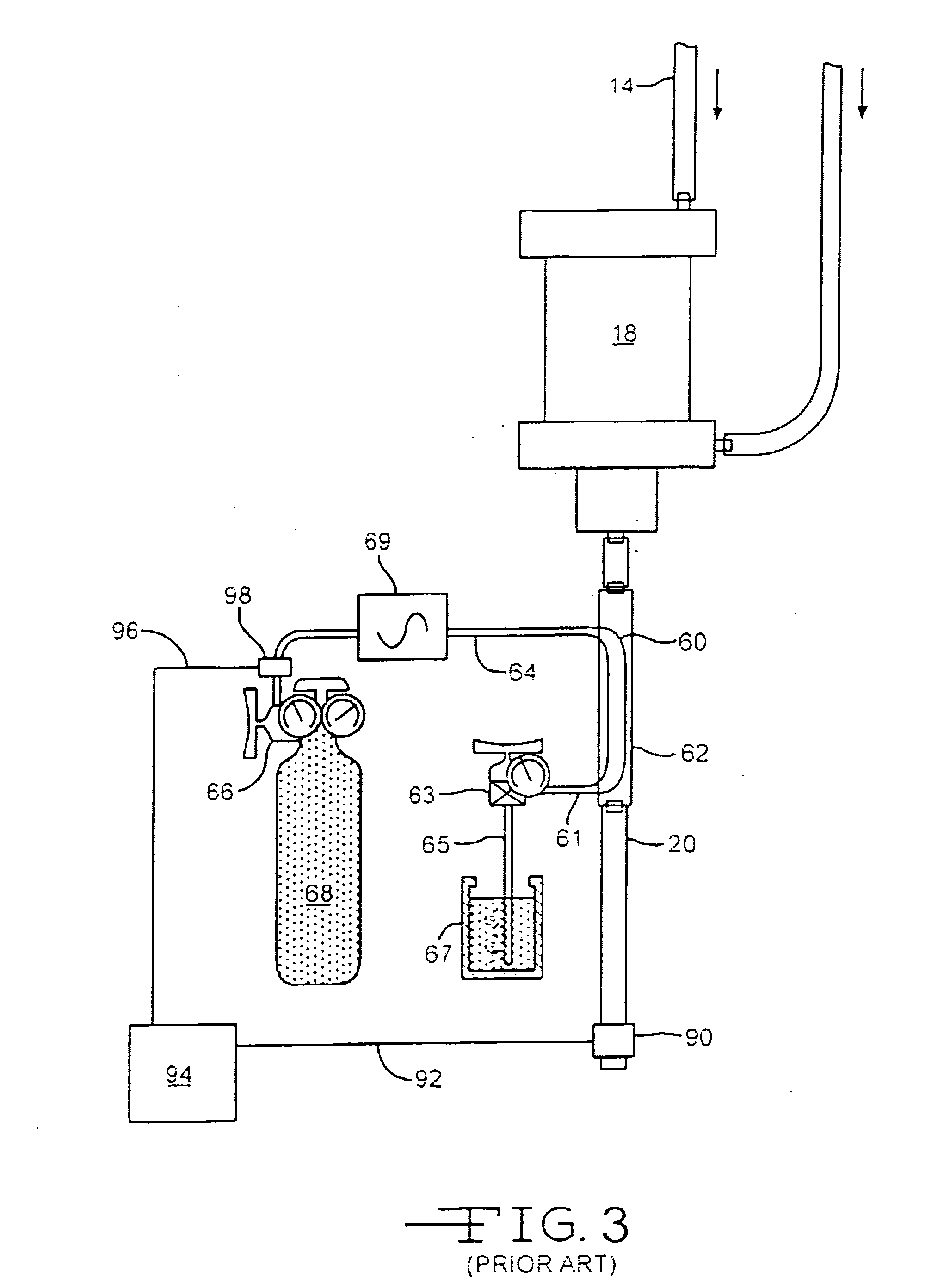
Hemostatic wire guided bandage and method of use
Some embodiments of the invention provide an apparatus for achieving hemostasis in a puncture tract that is created during a medical procedure. The puncture typically extends from the epidermis to the vasculature in a living organism. In some embodiments, the apparatus includes (1) a plug for subcutaneous placement within the puncture tract, and (2) a delivery mechanism for delivering and maintaining the plug within the puncture tract until hemostasis is achieved. The apparatus also includes in some embodiments a lubricious sheath that is placed around the plug to facilitate the insertion of the plug into the puncture tract.
Owner:NAT BOATLOADING LLC
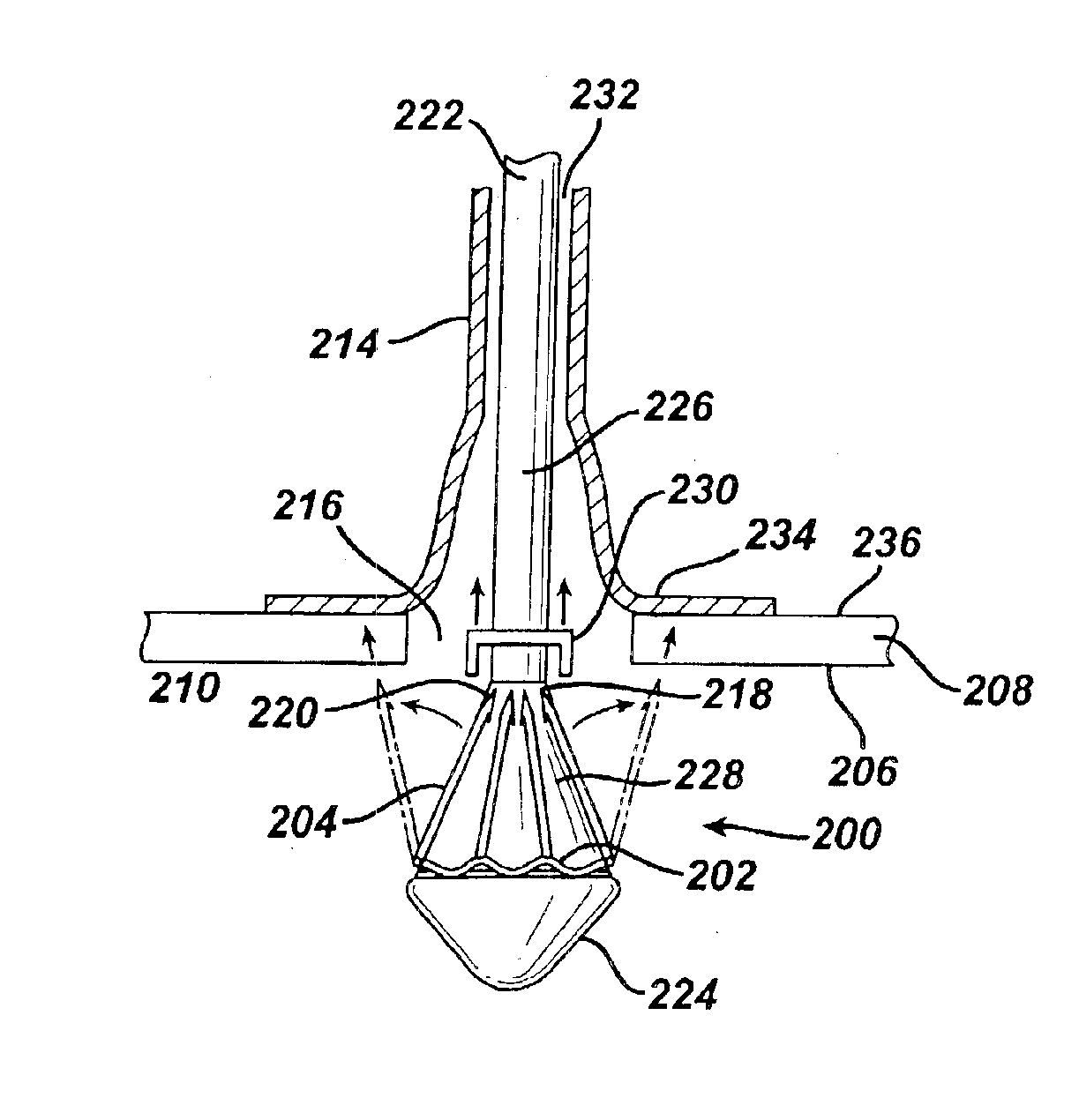
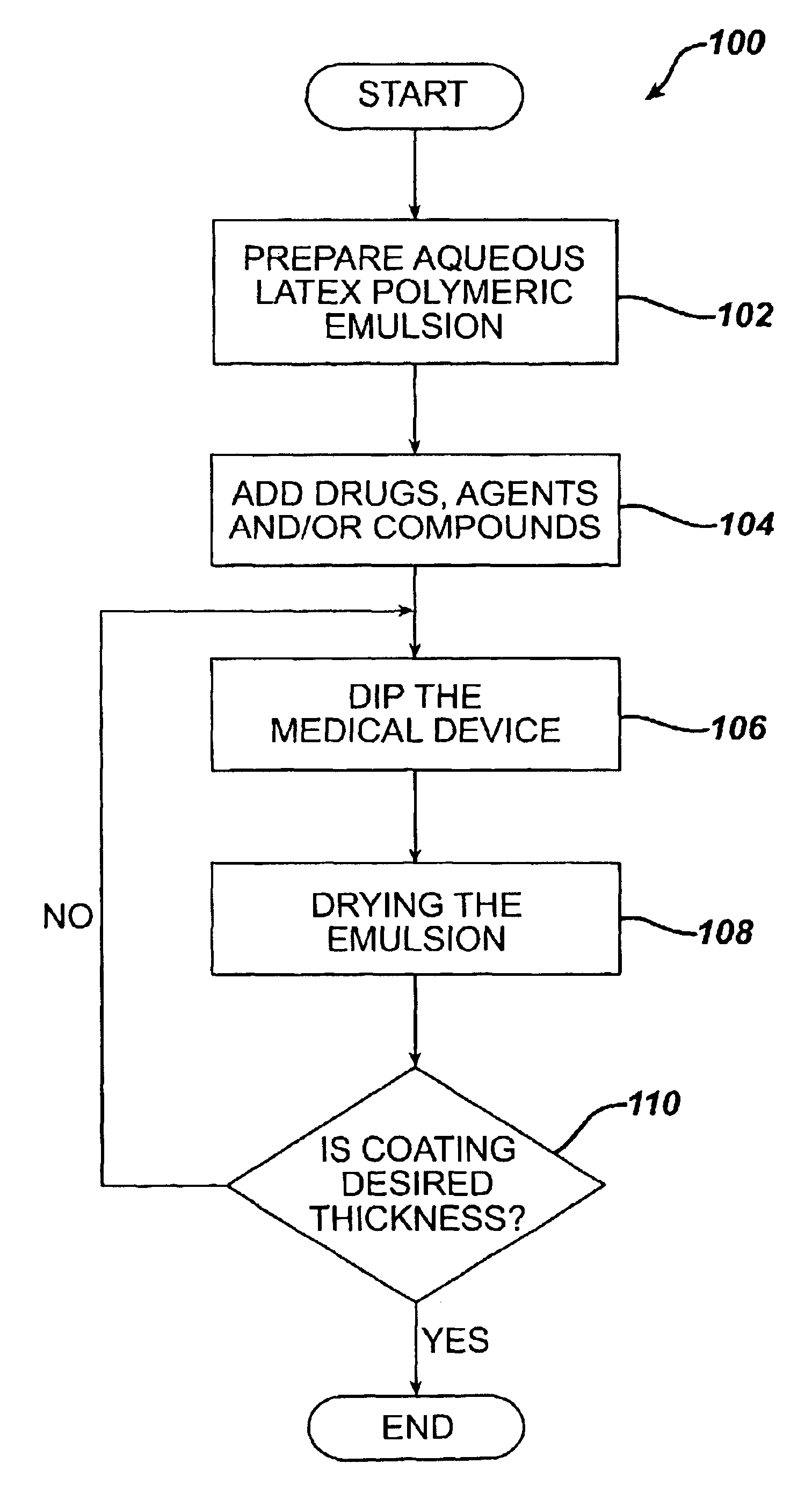
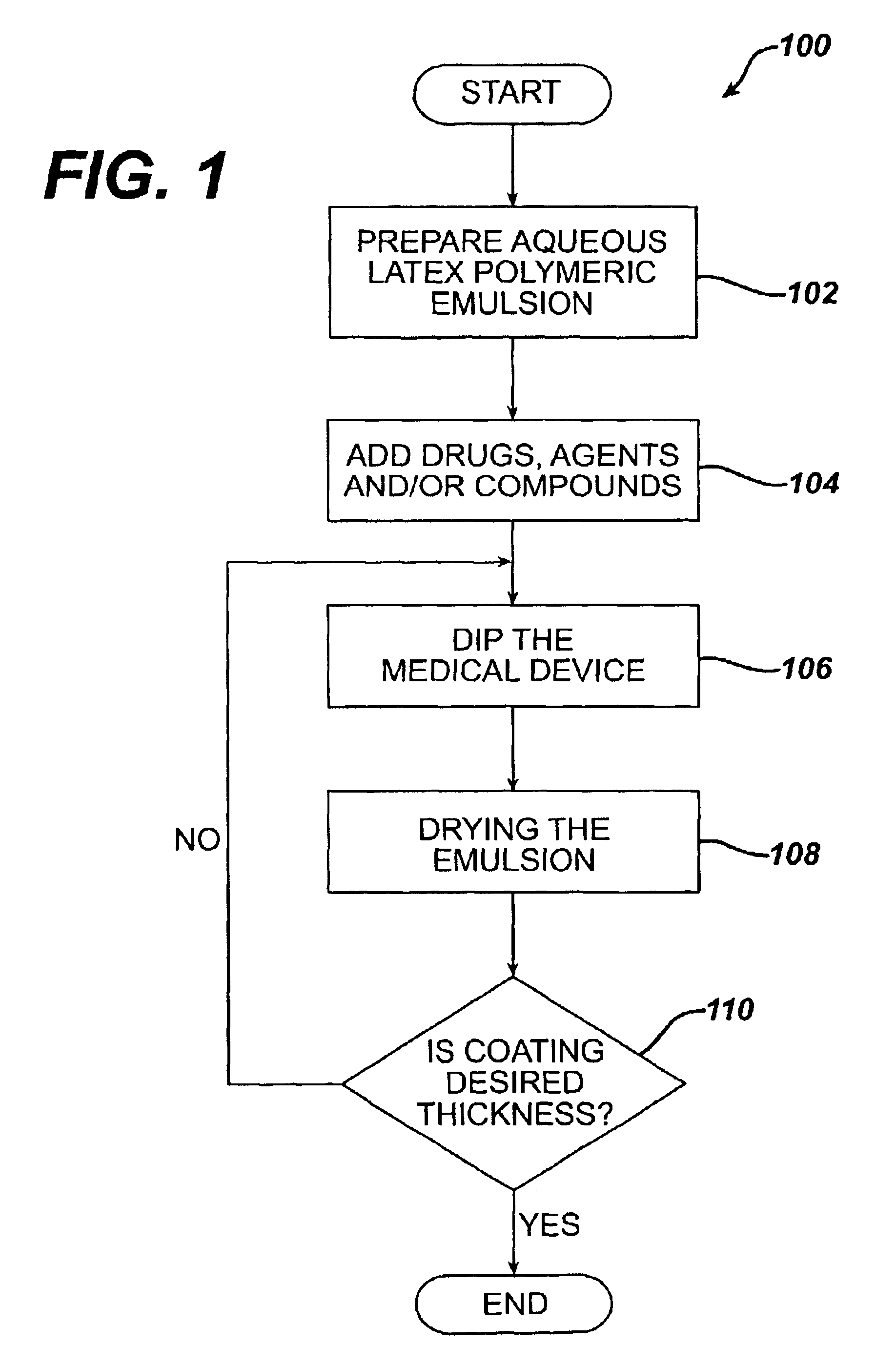
Method for coating medical devices
InactiveUS6919100B2Safe and efficient and effective processSimple and complex configurationOrganic active ingredientsImpression capsBiological bodyOrganic solvent
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism or to treat a particular condition. A dip coating process is utilized to minimize waste. An aqueous latex polymeric emulsion is utilized to coat any medical device to a desired thickness by allowing for successive dipping and drying cycles. In addition, aqueous latex polymeric emulsions pose less of a chance of the bridging phenomenon associated with organic solvent based polymers.
Owner:WYETH LLC
Medical devices, drug coatings and methods of maintaining the drug coatings thereon
InactiveUS20060222756A1Reduce drug toxicityGood curative effectSuture equipmentsOrganic active ingredientsBiological bodyReady to use
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:WYETH LLC
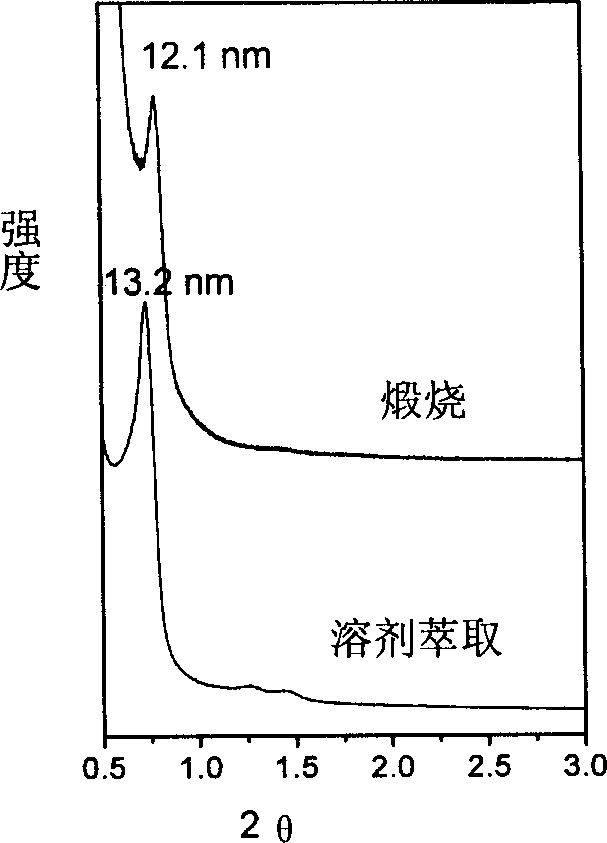
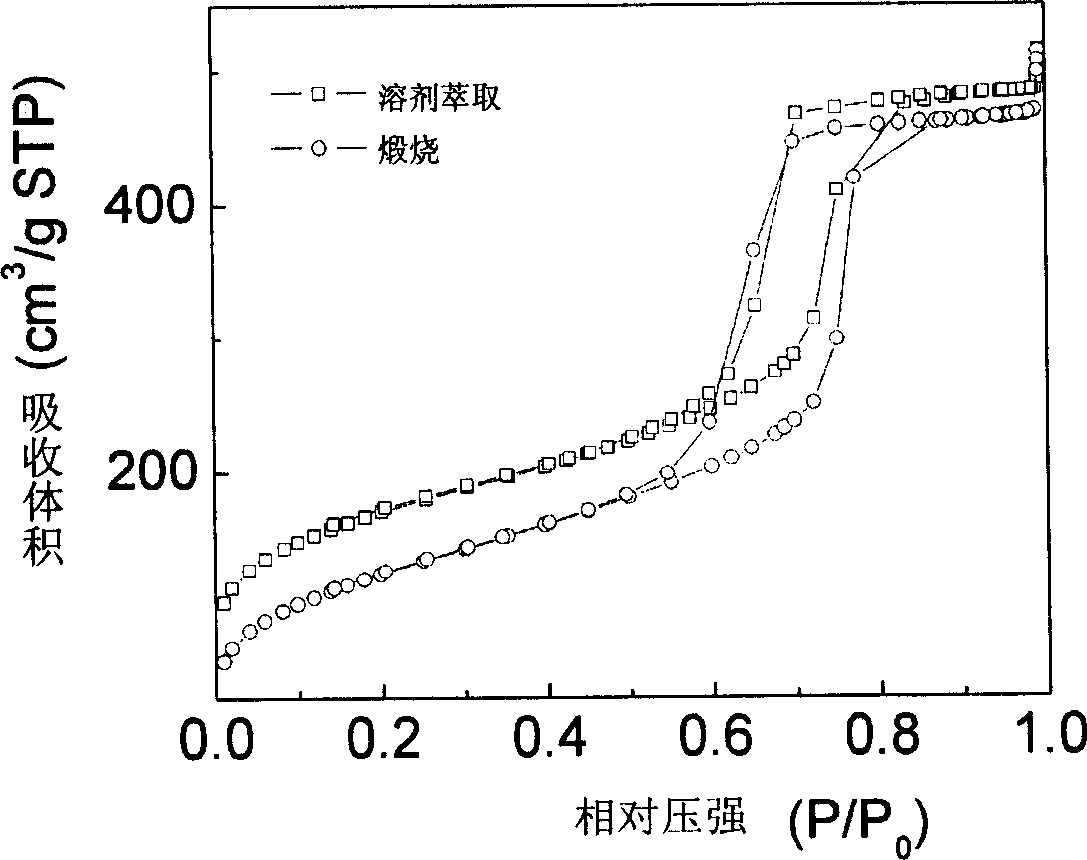
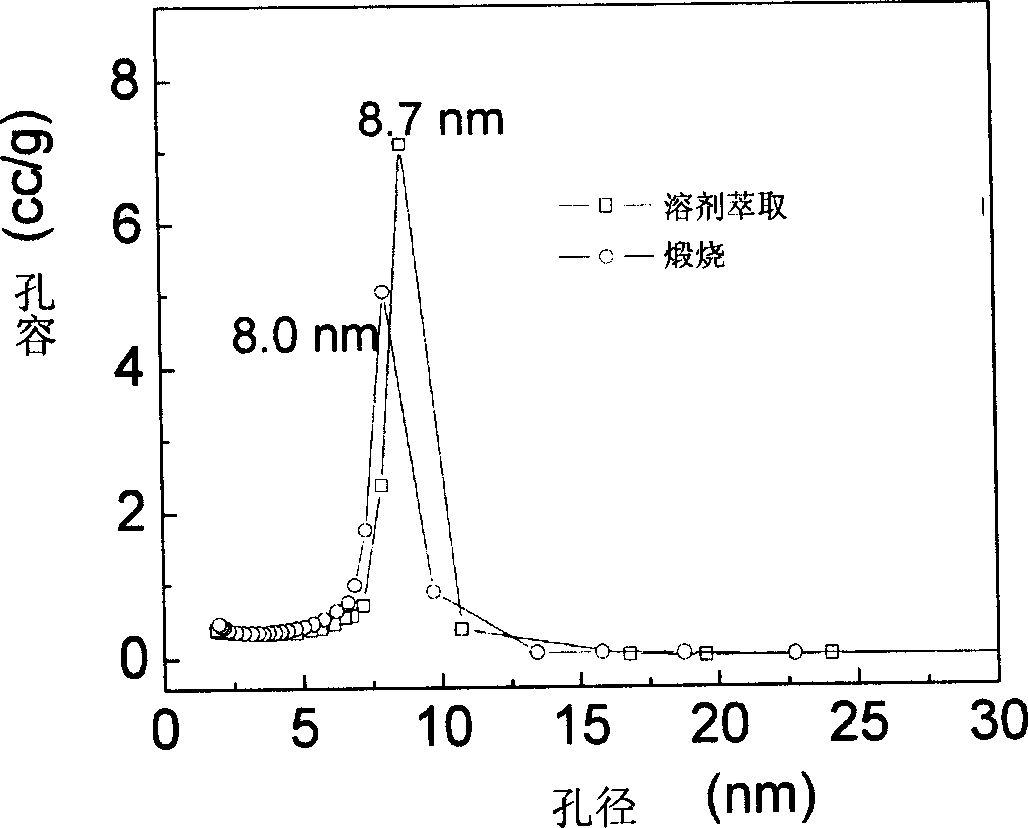
Method for preparing mesoporous carbon material
The present invention belongs to the field of material preparing technology. By means of sol-gel technology, organic high molecule and silicon source are led into a surfactant self-assembling reaction system so as to prepare high ordered mesoporous high molecule / silica and carbon / silica composite material and ordered mesoporous carbon material through organic-organic competition, inorganic-inorganic competition, organic-inorganic competition, cross-linking polymerization coordinate assembling and solvent volatizing self assembling. The prepared mesoporous carbon material has high order property, great specific surface area, great caliber and great porosity, as well as excellent electrochemical properties as super capacitor and cell material. It has also wide application foreground in catalysis, adsorption, biomolecule separation, and other fields.
Owner:FUDAN UNIV
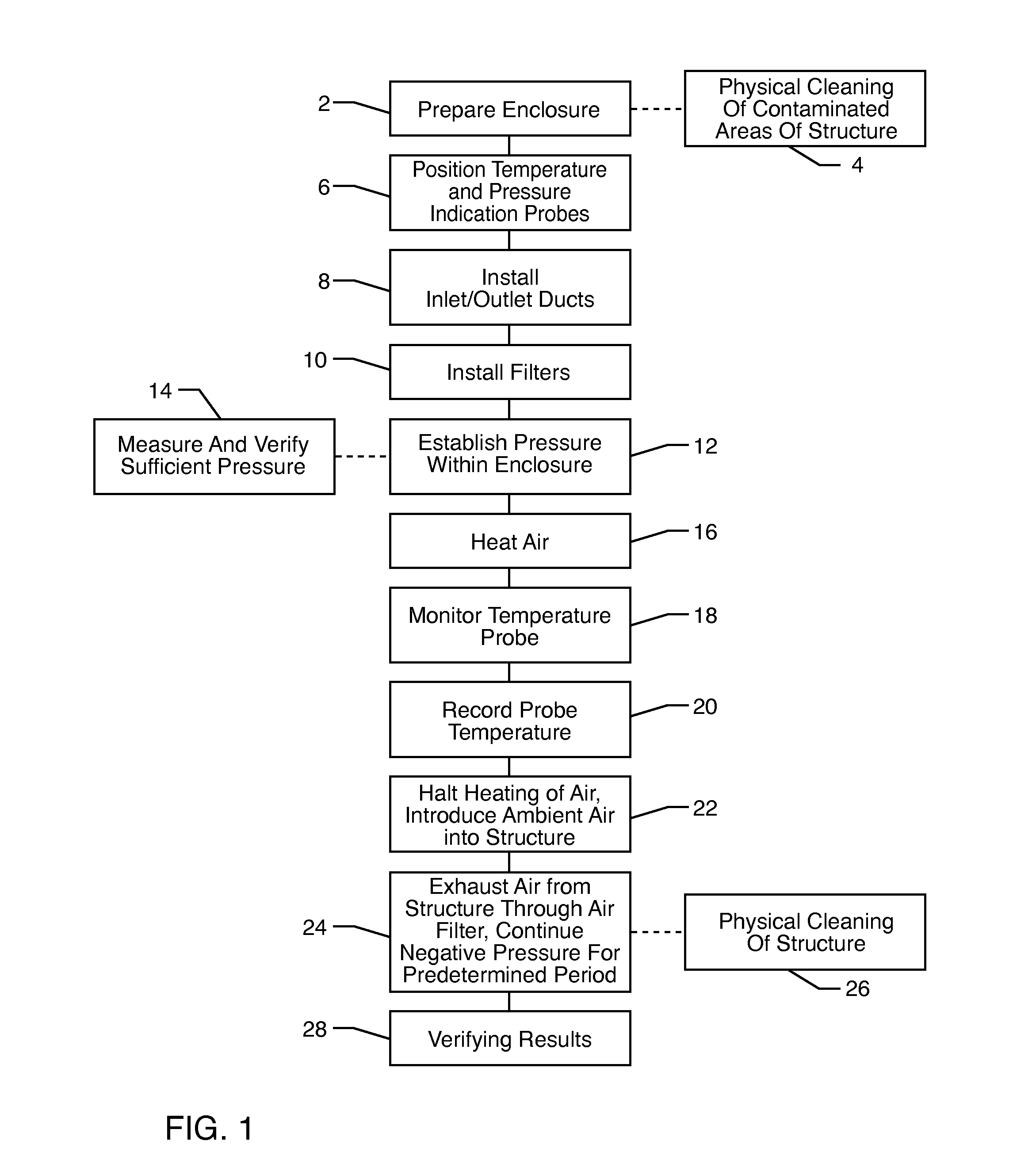
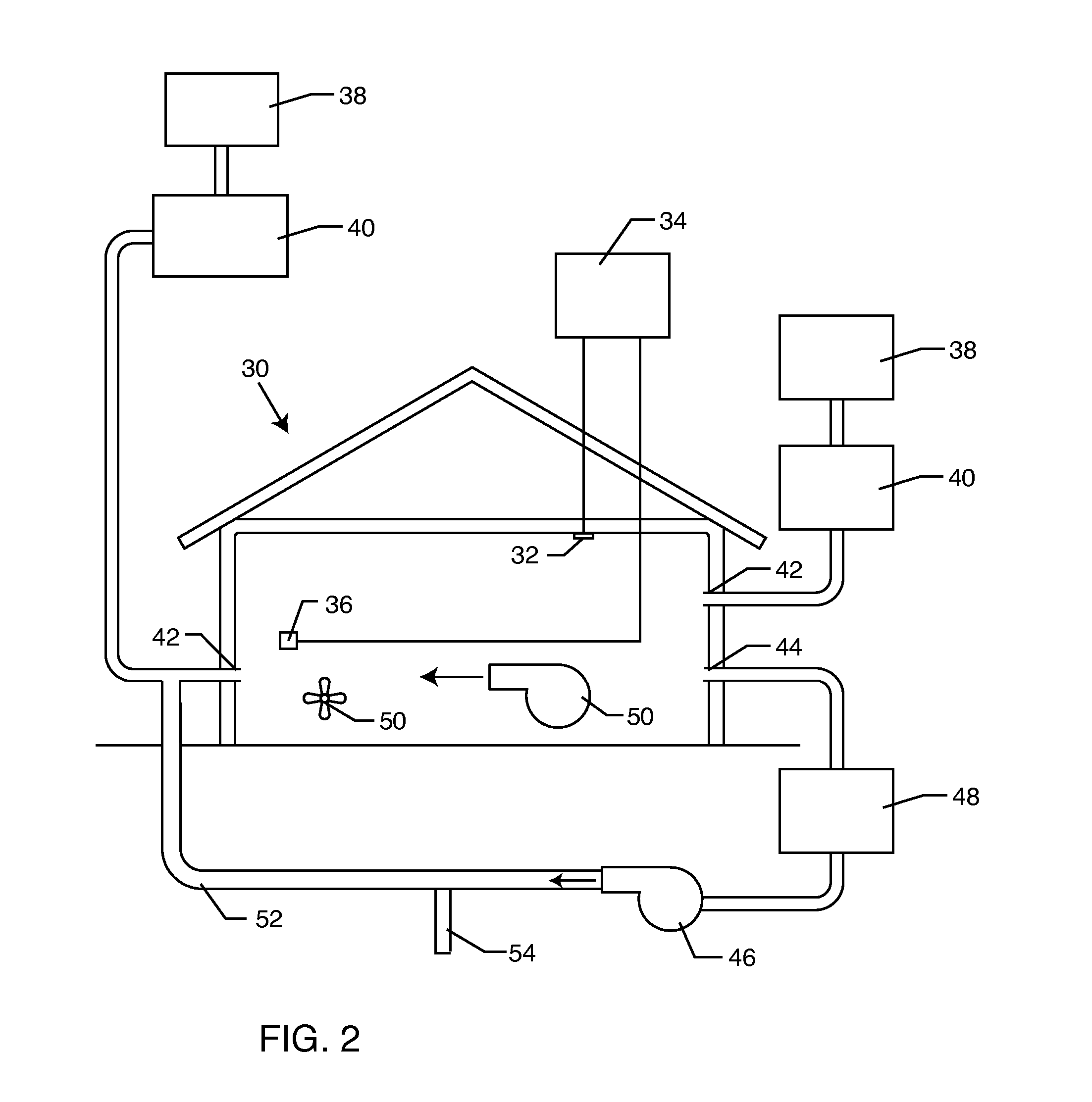
Method for removing or treating harmful biological organisms and chemical substances
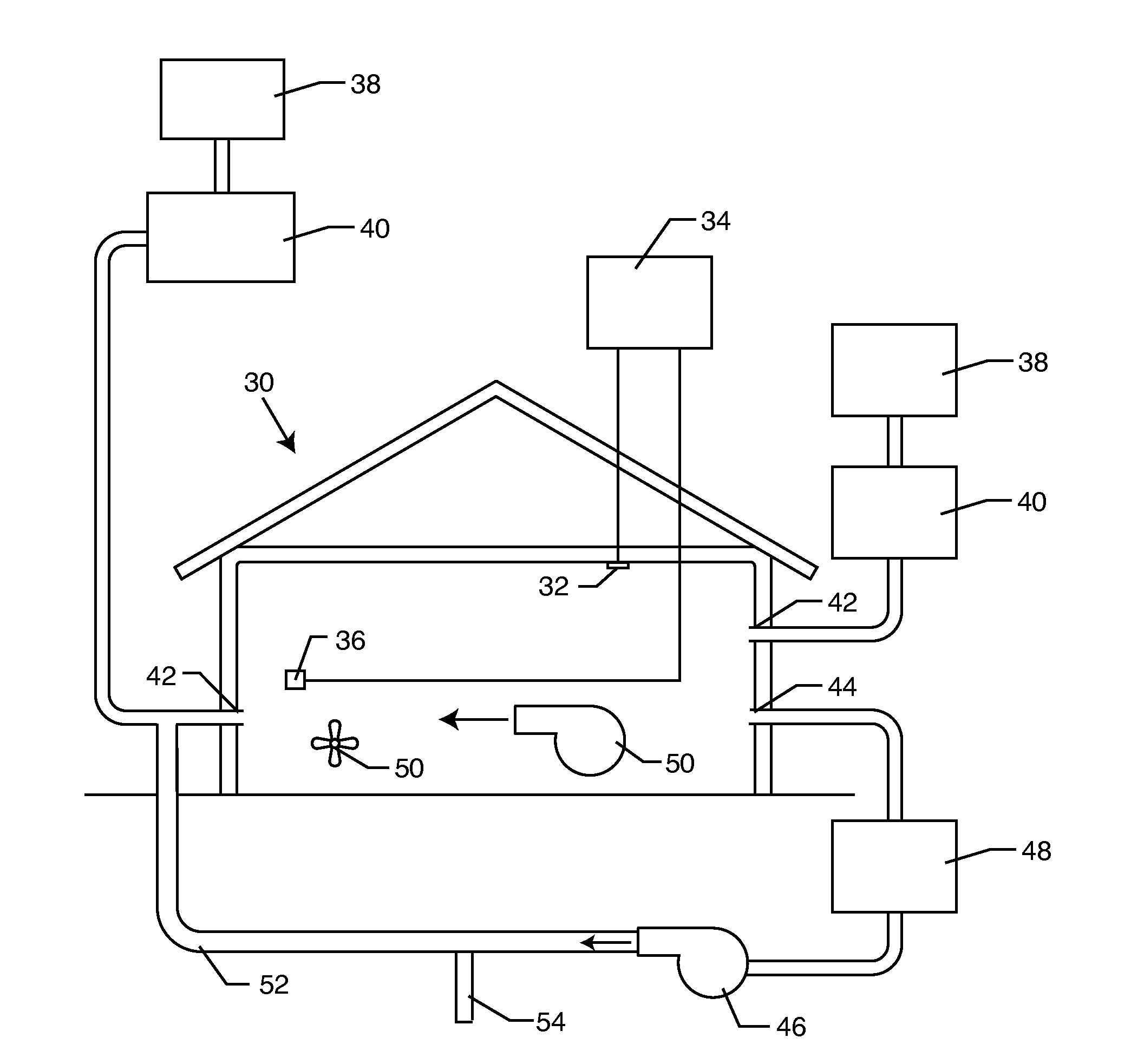
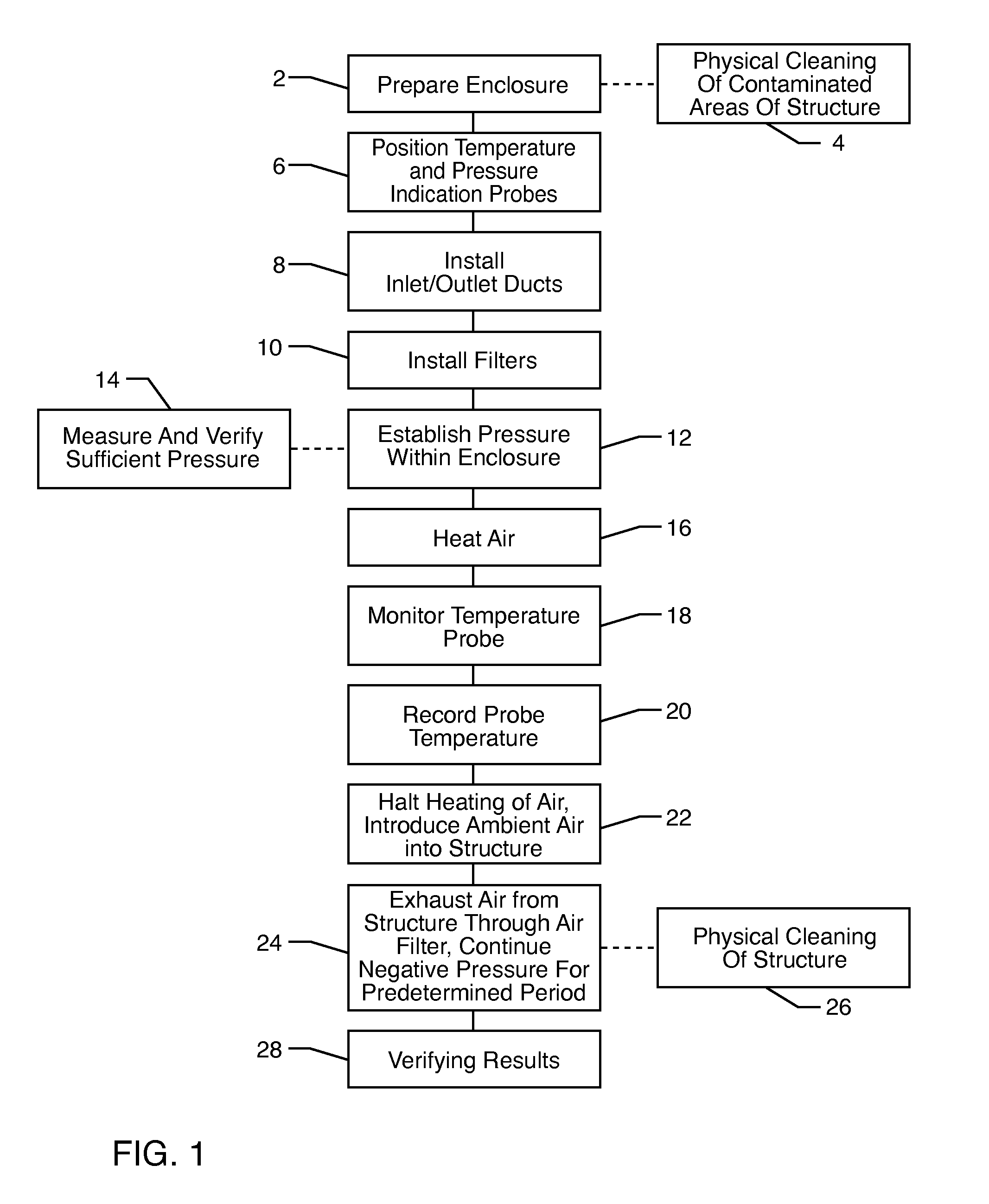
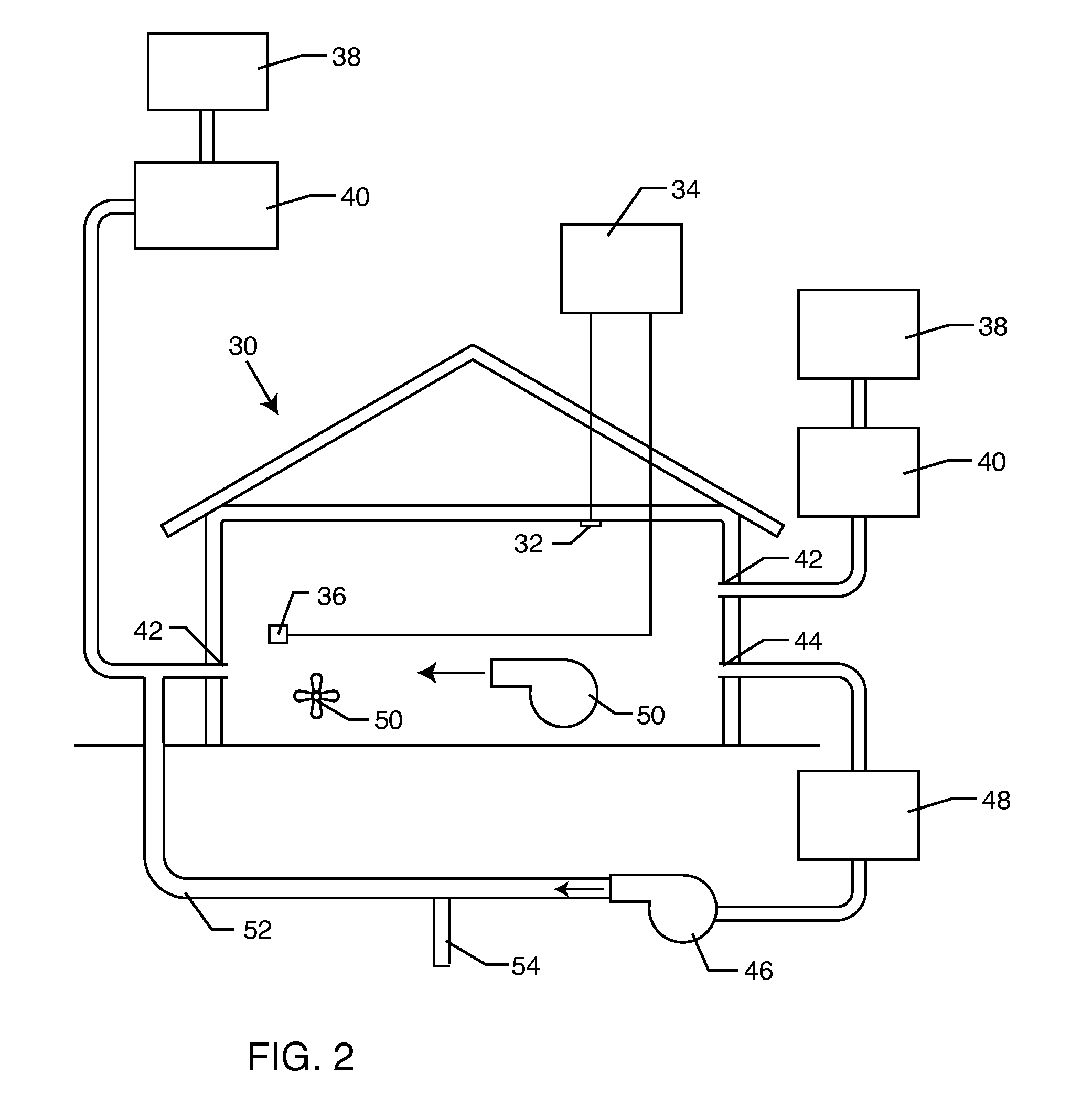
The present invention relates to methods of sanitizing structures, buildings, passenger occupiable vehicles, and other enclosed or enclosable spaces. More particularly, the present invention relates to a method for killing and / or removing pests and their allergens, bacteria, viruses, fungi, molds, volatile organic compounds and other dangerous substances, from such enclosures.
Owner:THERMAPURE
Fiber scaffolds for use creating implantable structures
A synthetic construct suitable for implantation into a biological organism that includes at least one polymer scaffold; wherein the at least one polymer scaffold includes at least one layer of polymer fibers that have been deposited by electrospinning; wherein the orientation of the fibers in the at least one polymer scaffold relative to one another is generally parallel, random, or both; and wherein the at least one polymer scaffold has been adapted to function as at least one of a substantially two-dimensional implantable structure and a substantially three-dimensional implantable tubular structure.
Owner:NFS IP HLDG LLC
Radioprotective compound coating for medical devices
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Also, the devices may be modified to promote endothelialization. Other compounds may include those that prevent damage from ionizing radiation. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:OHARA MICHAEL D
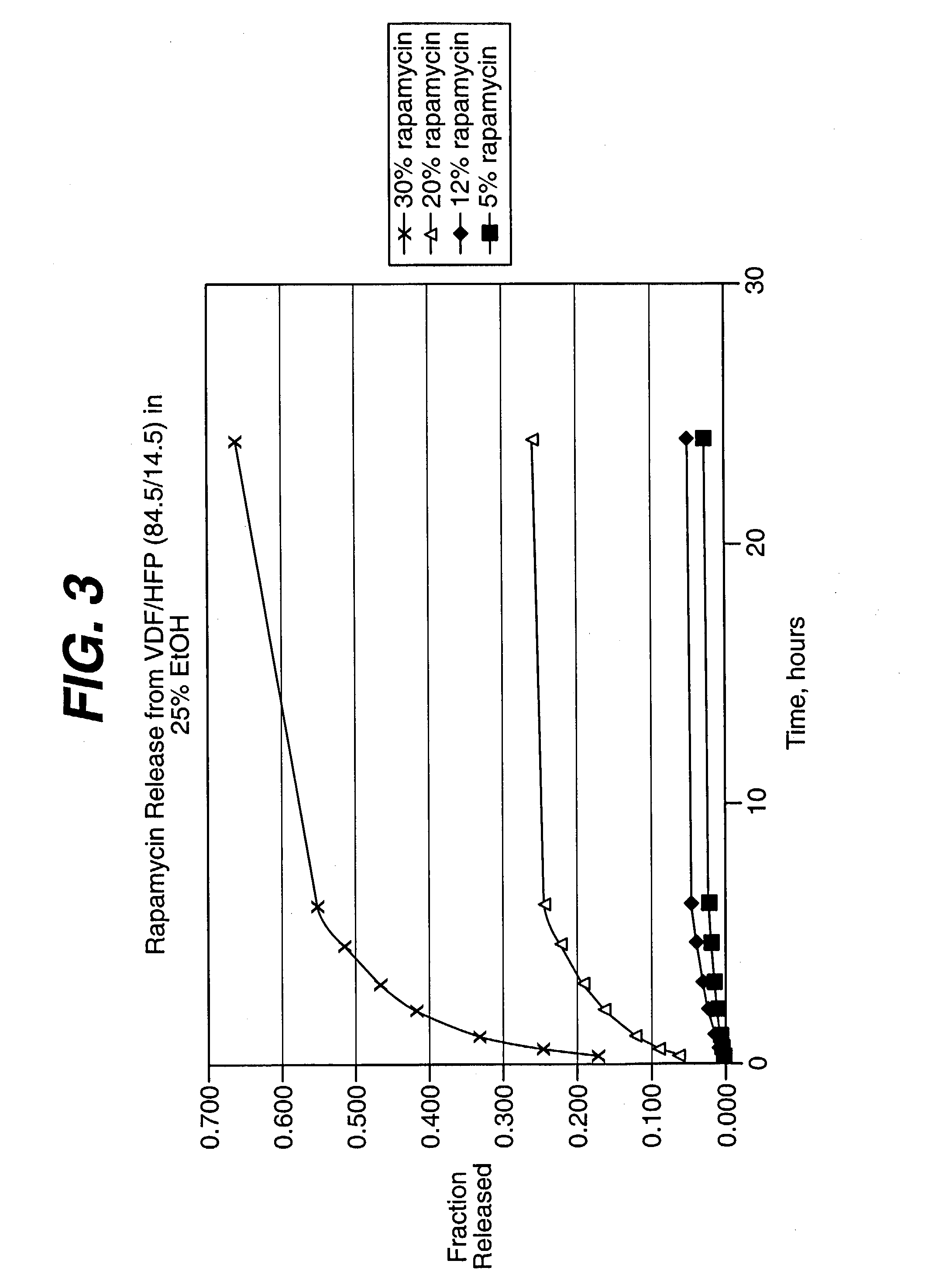
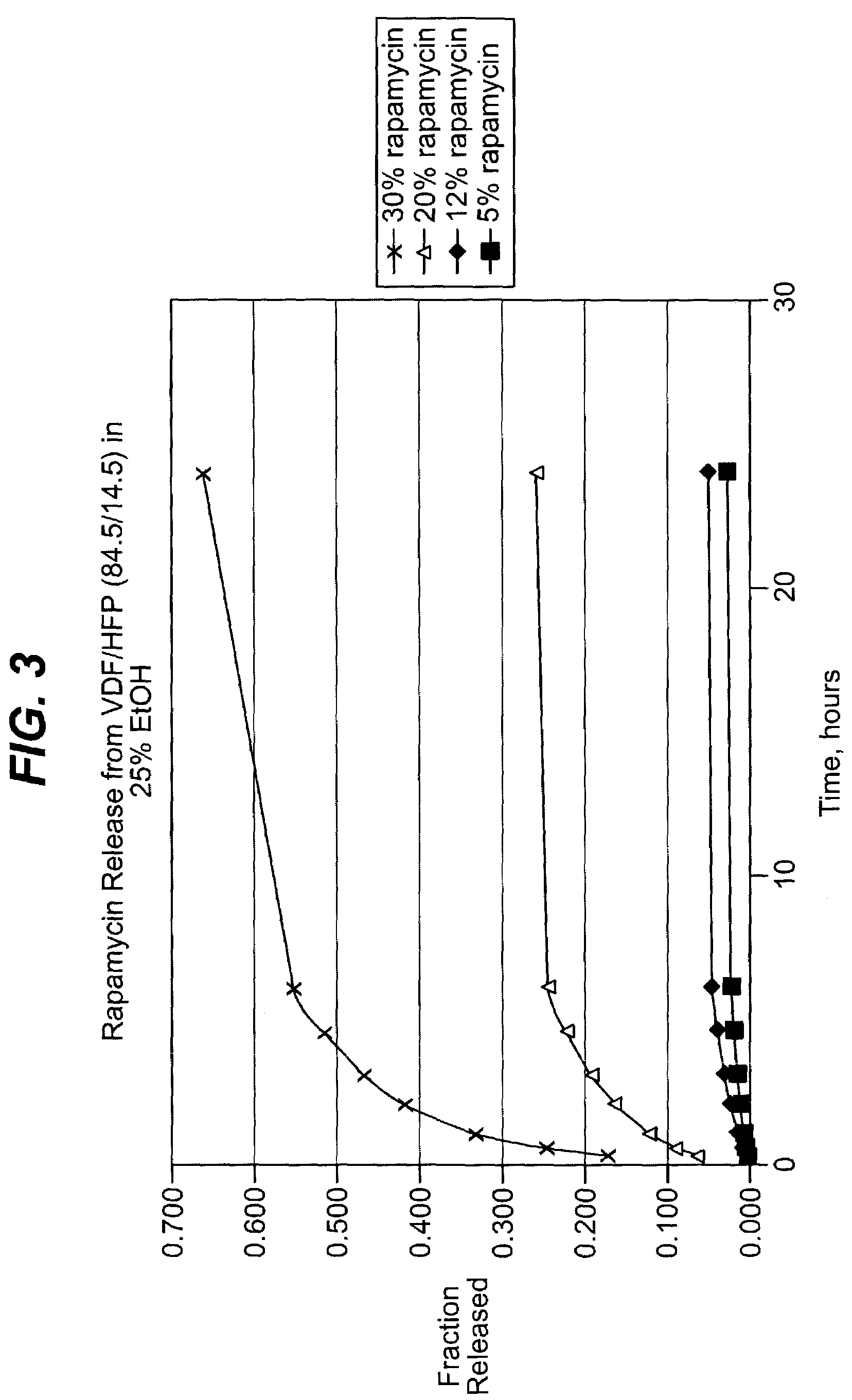
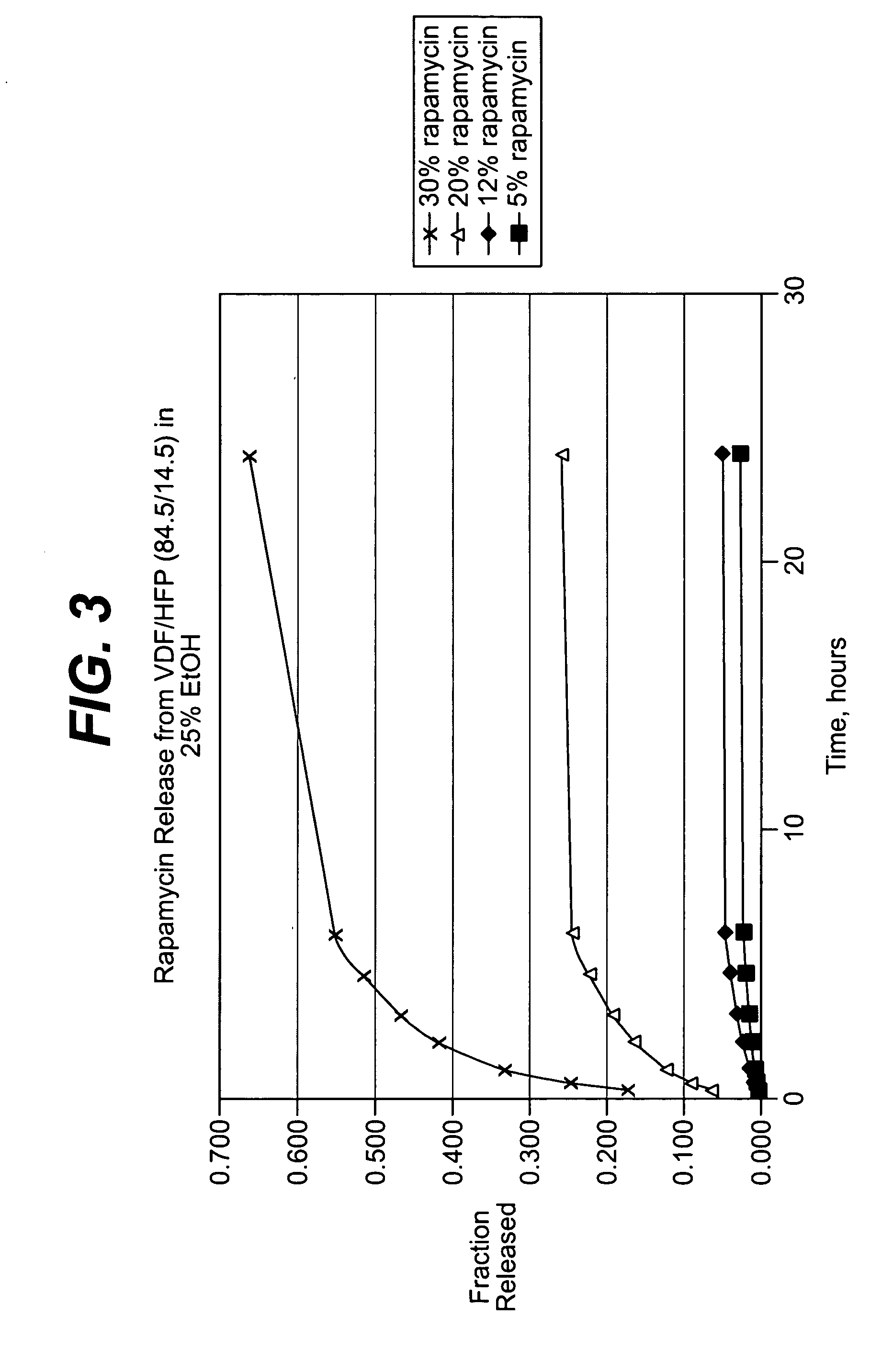
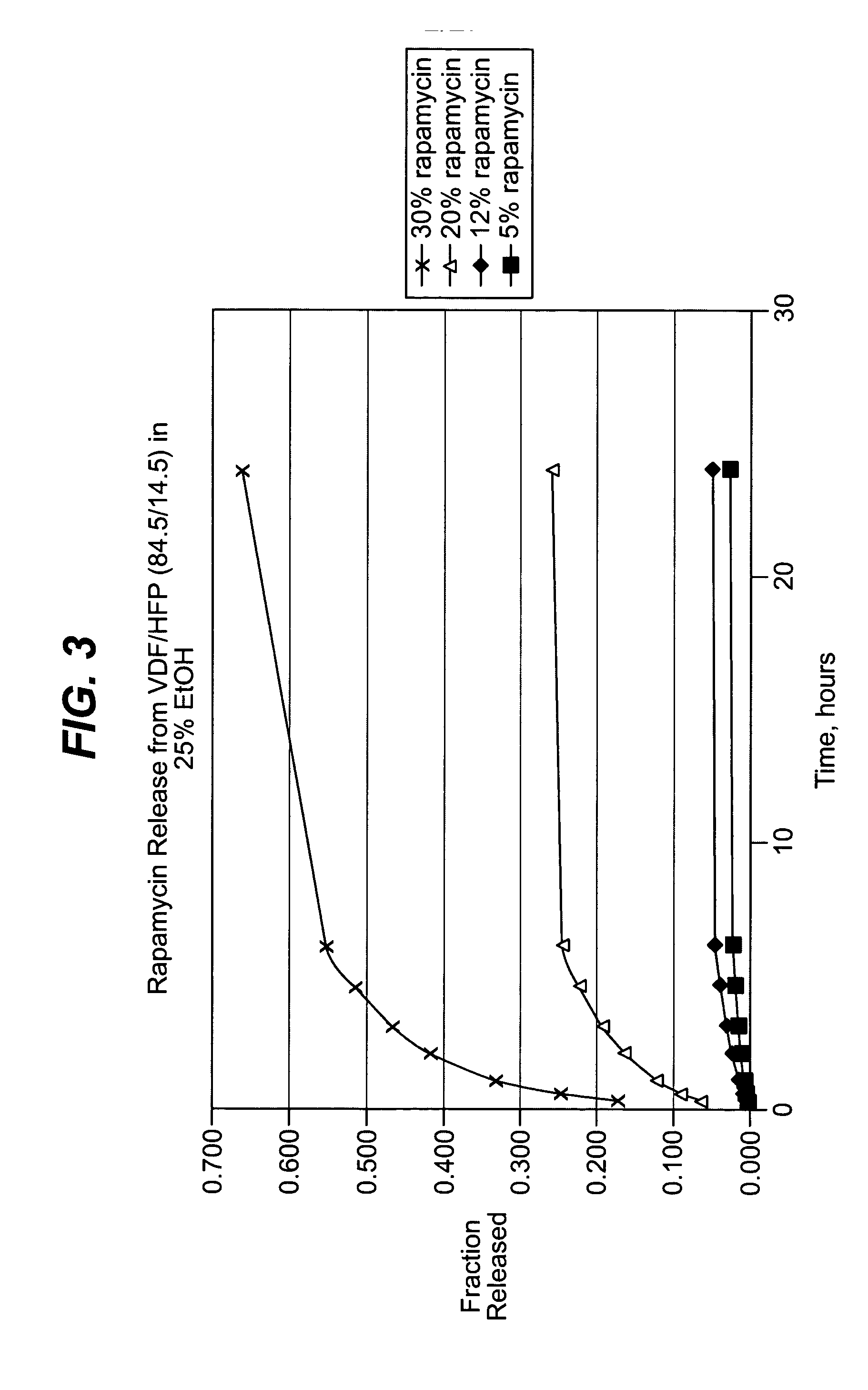
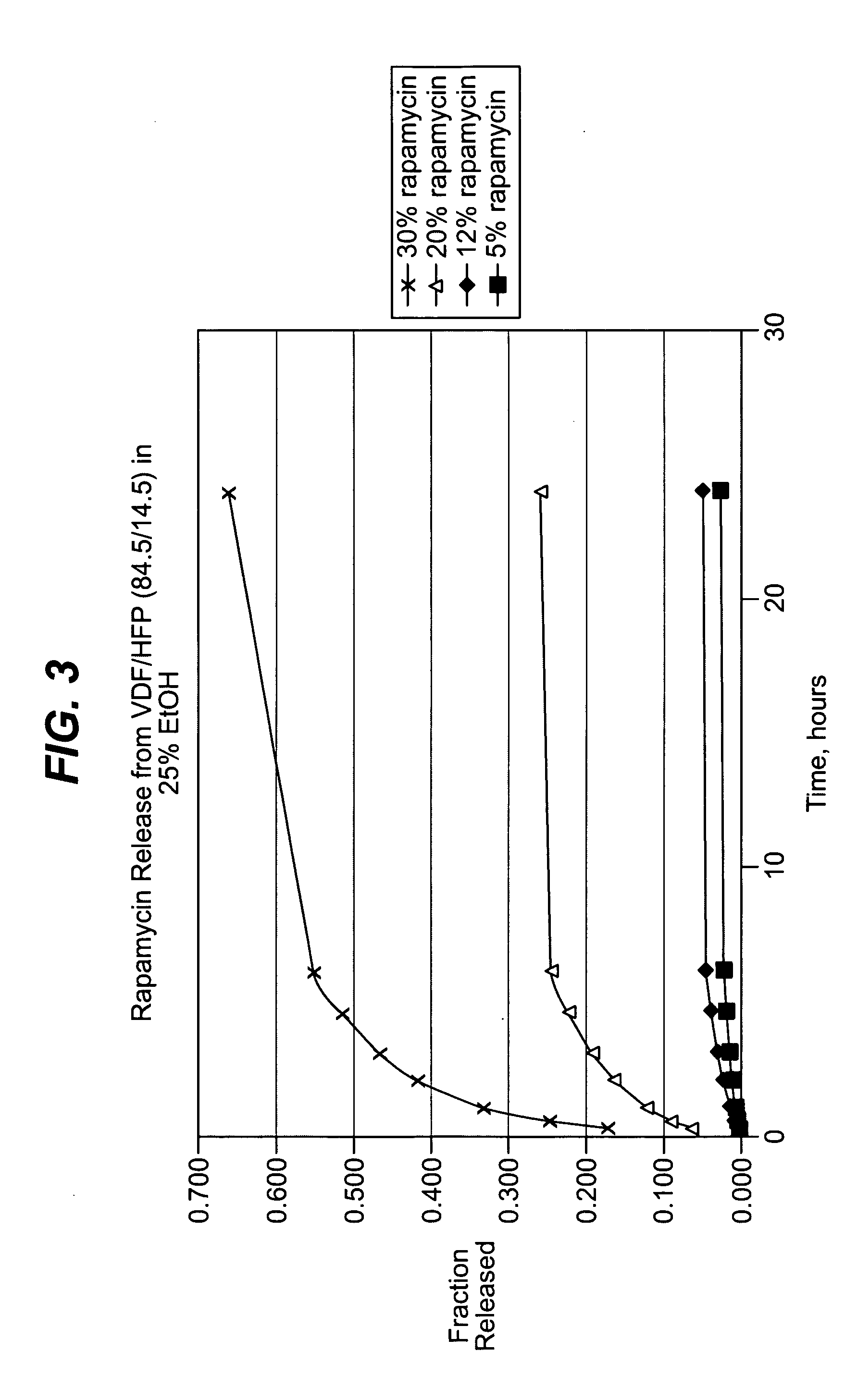
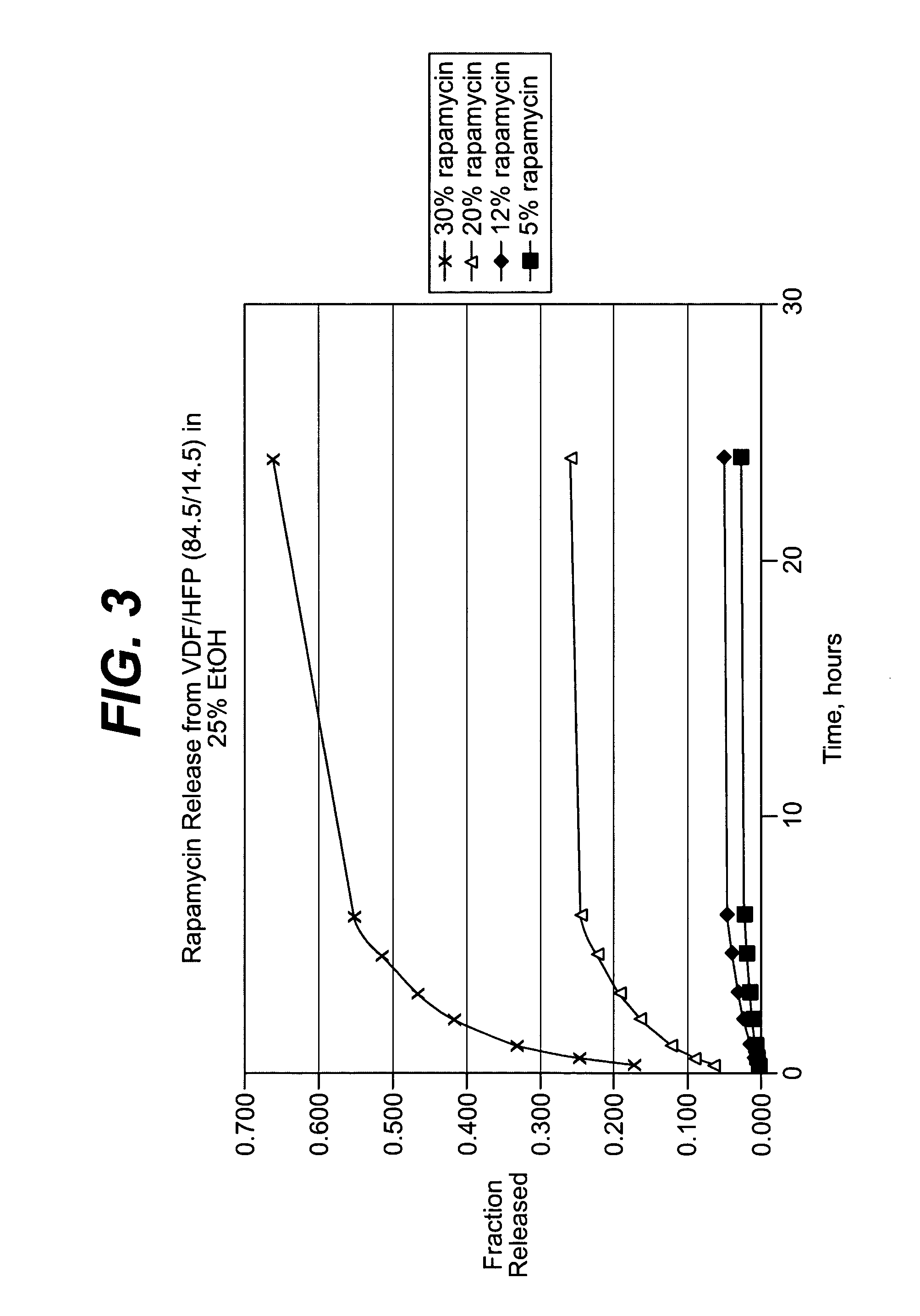
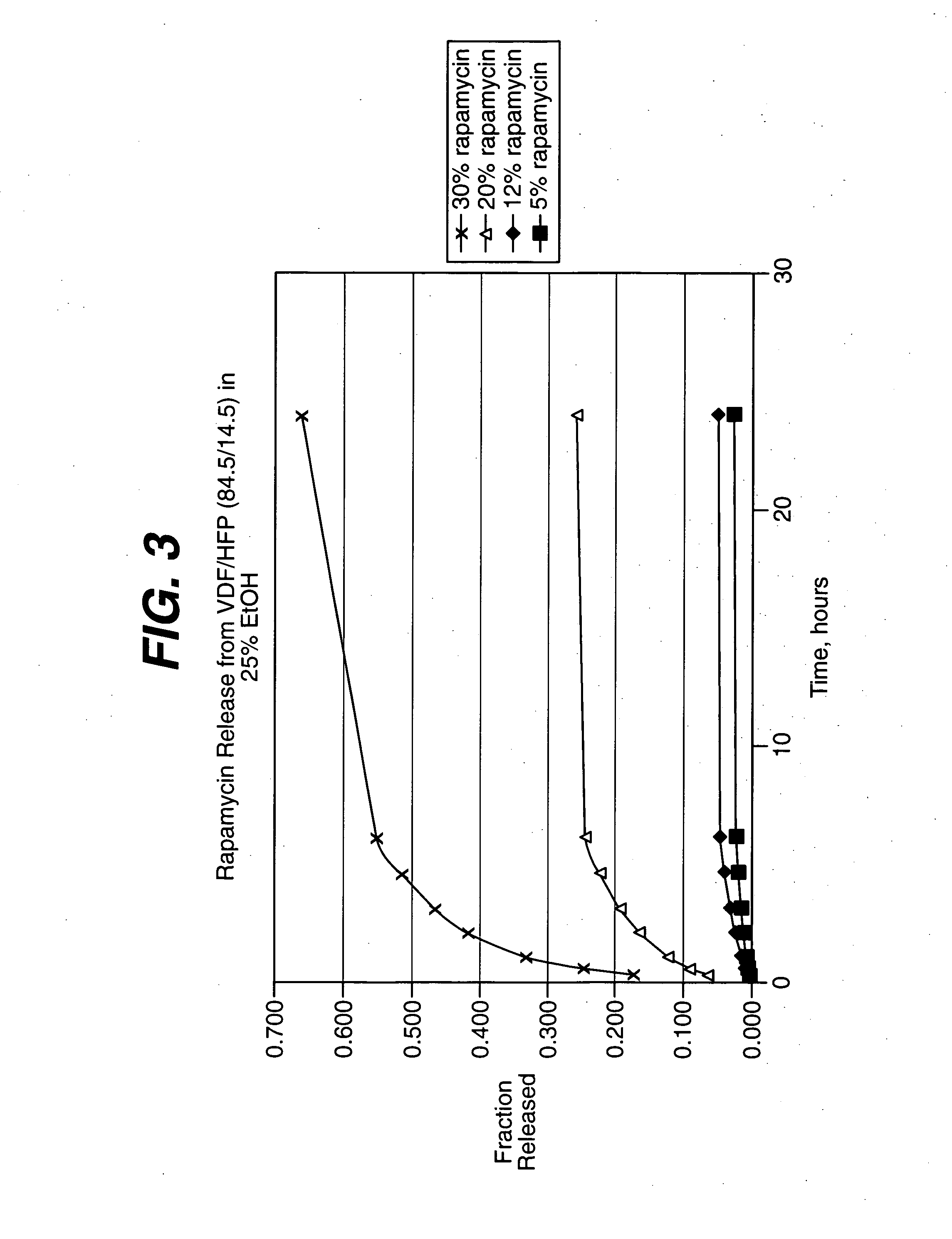
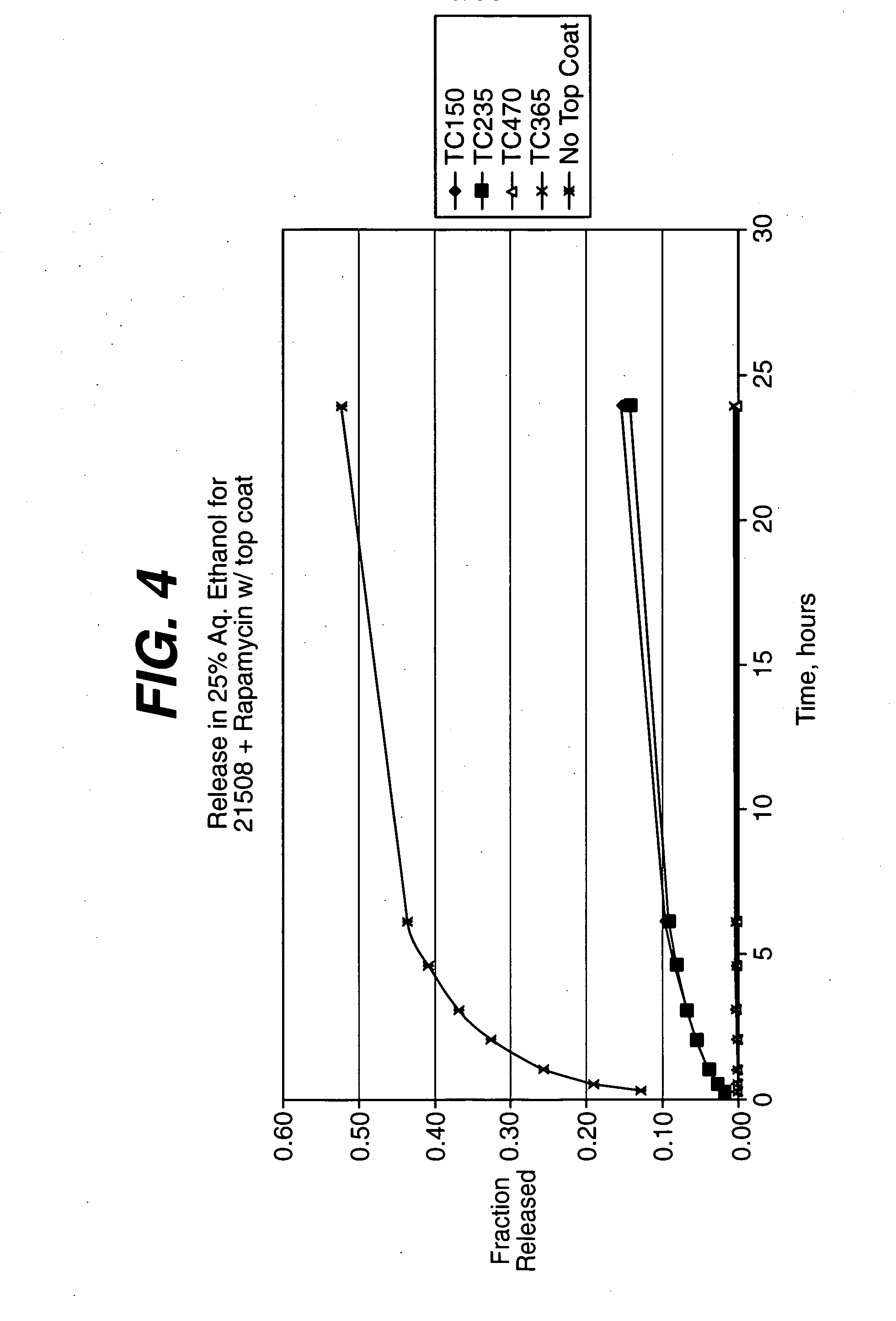
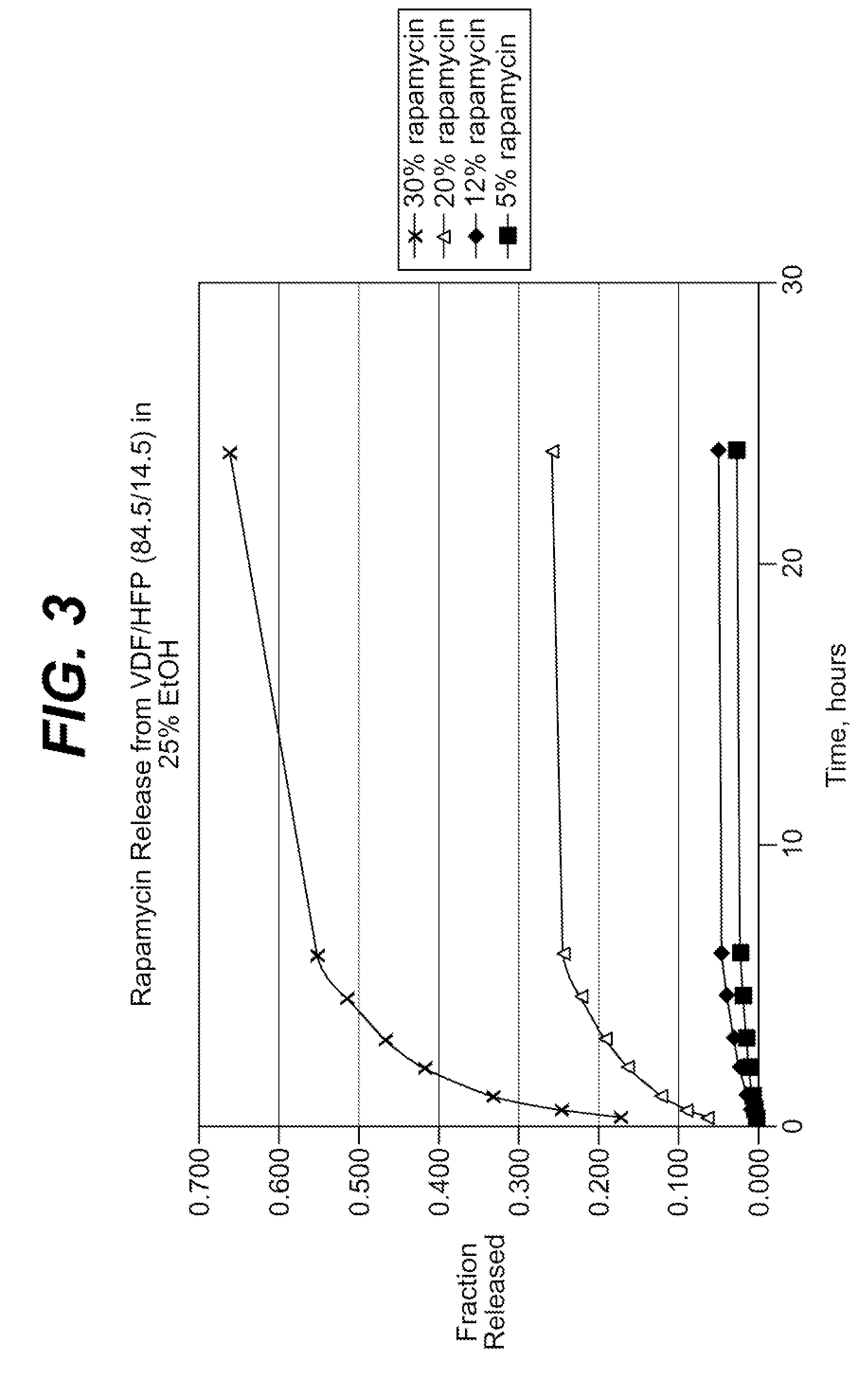
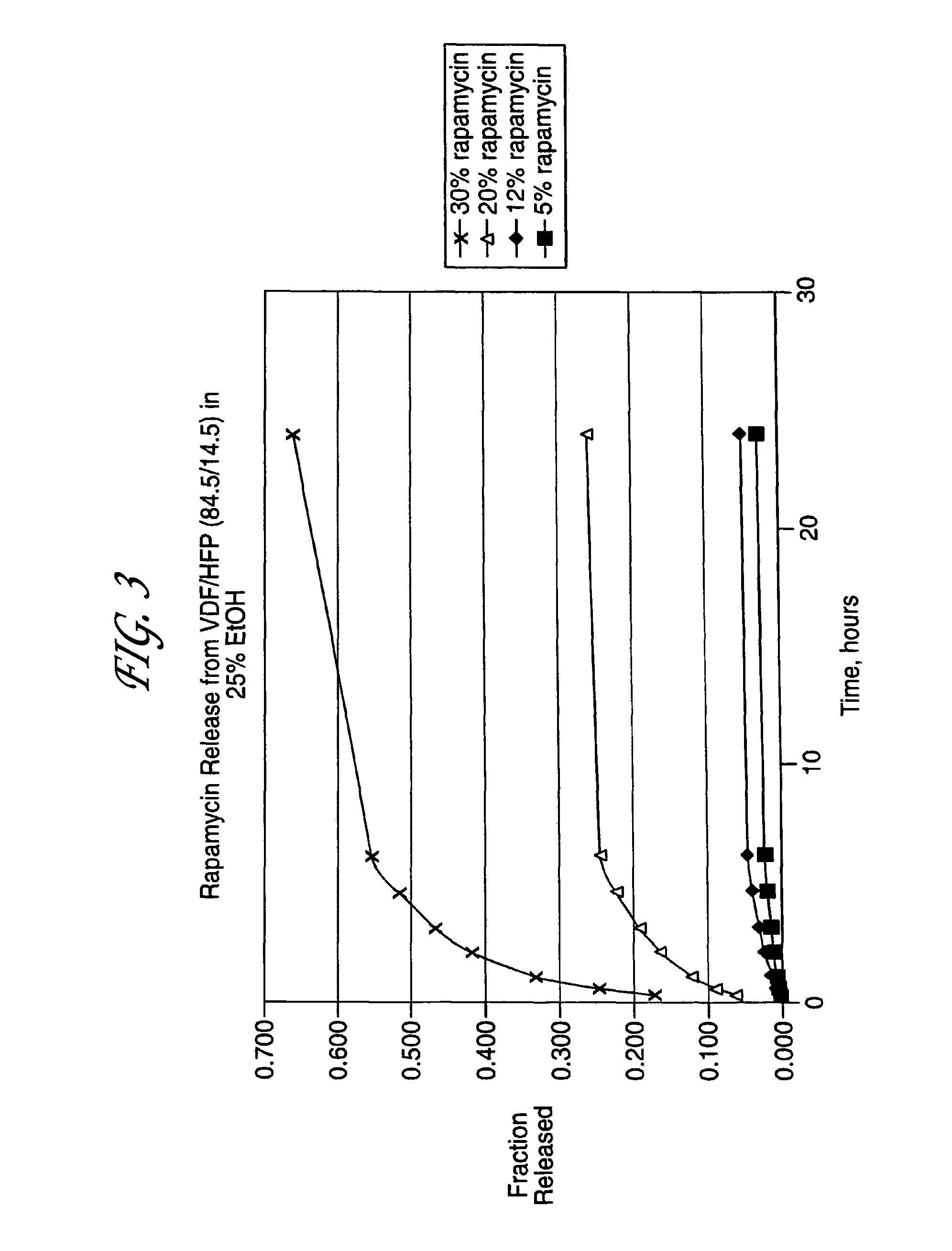
Local vascular delivery of cladribine in combination with rapamycin to prevent restenosis following vascular injury
ActiveUS20050182485A1Minimize potential risk of damageReduce frictionSuture equipmentsOrganic active ingredientsPercent Diameter StenosisBlood vessel
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
Antithrombotic coating for drug eluting medical devices
InactiveUS20070048350A1Improve surface adhesionImprove biological activityBiocideAntipyreticThrombusMedical treatment
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:WYETH LLC
Layer - by - layer stereocomplexed polymers as drug depot carriers or coatings in medical devices
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the prevention of thrombosis. The drugs, agents, and / or compounds may also be utilized to treat specific disorders, including vulnerable plaque, and atherosclerosis in type 2 diabetic patients. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Various materials and coating methodologies may be utilized to maintain the agents or compounds on the medical device until delivered and positioned.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Hen green complex feed additive and preparation method thereof
InactiveCN101176510ANo side effectsNo drug resistanceFood processingAnimal feeding stuffAnti stressSide effect
The invention discloses a feed additive and a preparation craft, in particular to a green feed additive for layers and the preparation craft, which is prepared by the mixure of Chinese medicine chemical additive, micro ecology preparation, Vitamin class, trace elements, methionine, chlorination choline, ethoxybenzoylaminoquinoline, rice husk powder and zeolite. The invention has the advantages of nontoxic side effect, no residue, no antibiotic nature, improvement of the laying intensity of layers and the quality of eggs, and enhancement of organism immunity and the anti-stress ability of the organic body.
Owner:辽宁省农业科学院草牧业研究所
Method for removing or treating harmful biological organisms and chemical substances
The present invention relates to methods of sanitizing structures, buildings, passenger occupiable vehicles, and other enclosed or enclosable spaces. More particularly, the present invention relates to a method for killing and / or removing pests and their allergens, bacteria, viruses, fungi, molds, volatile organic compounds and other dangerous substances, from such objects or enclosures.
Owner:THERMAPURE
Medical Devices, Drug Coatings and Methods for Maintaining the Drug Coatings Thereon
InactiveUS20080051885A1Reduce drug toxicityGood curative effectStentsSurgeryBiological bodyCompound (substance)
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CORDIS CORP
Method for realizing concentrated searching on handheld learning terminal
InactiveCN102122286AAchieve sharingRealize rationalityElectrical appliancesSpecial data processing applicationsData memoryKnowledge management
The invention relates to a method for realizing concentrated searching on a handheld learning terminal, in particular to a method for realizing concentrated searching including test searching, extracurricular knowledge searching and knowledge explanation searching on the handheld learning terminal based on the sharing of learning resources in multi-functional areas. A compressed and unified learning resource information database is memorized in a memory of the handheld learning terminal, and the learning resource information database is an organic body formed by combining a plurality of relevant and complemented data memory units of a knowledge concept element database, a learning assessment resource repository, a knowledge explanation resource repository, a test relevance database and an extracurricular knowledge database. By the method provided by the invention, the effective range for searching on the handheld learning terminal by a user can be no longer limited by a test searching packet, and the user can perform test self-selection, individual test paper grouping, learning diagnosis and intelligent testing by combining other relevant learning functions, so that individual learning is realized really.
Owner:武汉学优科技有限公司
Use of nitric oxide in the treatment and disinfection of biofilms
The administration of gaseous nitric oxide as a biocidal moiety is proffered as a de novo treatment in the control and eradication of biofilms. The present invention relates to the use or methods of application of exogenous nitric oxide gas (gNO) as a stand alone biocidal agent or in cohort with any or all adjunct vehicles in the control of biofilms generated by microbial organisms, i.e., bacteria, protozoa, amoeba, fungi etc. Further, the present invention introduces the concept of utilization and methods of application of gaseous nitric oxide in control and eradication of biofilm forming microorganisms. Other embodiments include the use of a nitric oxide releasing material to eradicate and-control the growth of biofilms. Another embodiment includes the use of a gaseous nitric oxide releasing material packaged in an air-tight container with a medical device to prevent the growth of biofilm on the medical device.
Owner:PULMONOX TECH
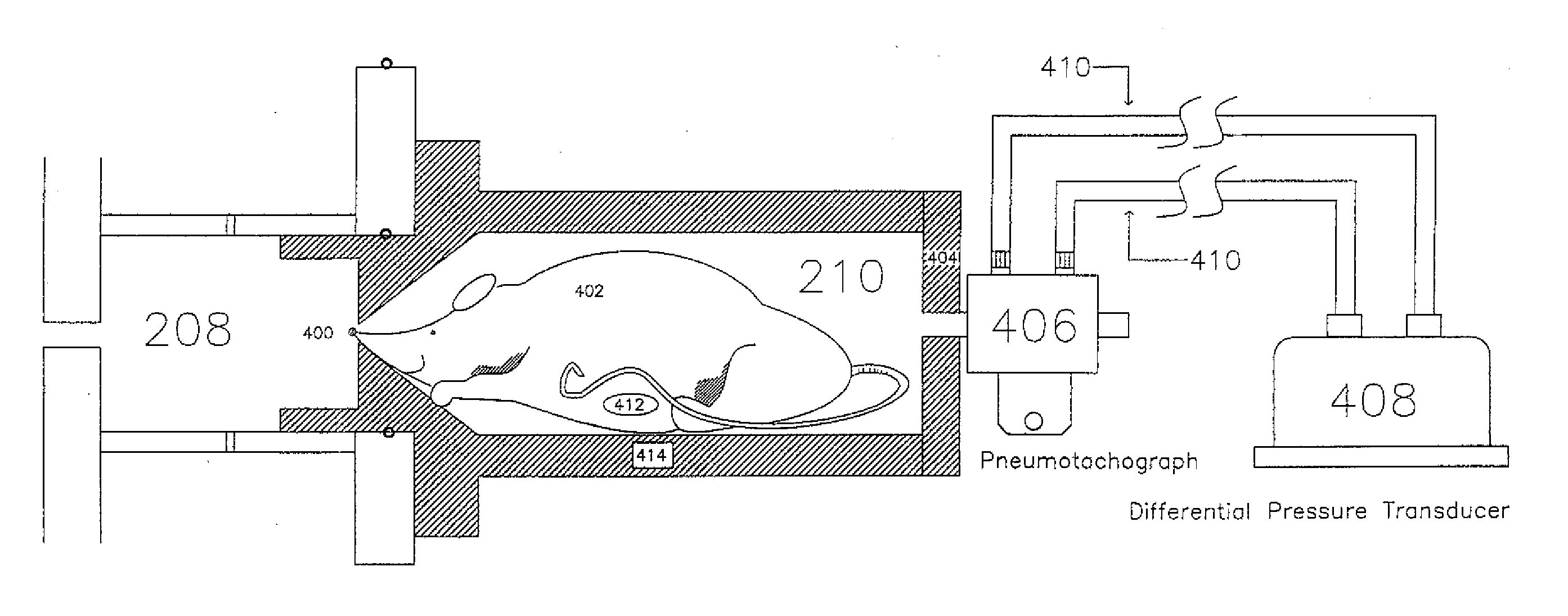
Automated Inhalation Toxicology Exposure System and Method
In one embodiment, a method includes but is not limited to: conditioning an inhalent environment; exposing a first organism to the inhalent environment for a first-organism duration of time; and exposing a second organism to the inhalent environment for a second-organism duration of time. In addition to the foregoing, other method embodiments are described in the claims, drawings, and text forming a part of the present application. In one or more various embodiments, related systems include but are not limited to circuitry and / or programming for effecting the foregoing-referenced method embodiments; the circuitry and / or programming can be virtually any combination of hardware, software, and / or firmware configured to effect the foregoing-referenced method embodiments depending upon the design choices of the system designer. In one embodiment, a system includes but is not limited to: an inhalent manifold; a first independently-controllable exposure unit coupled to said inhalent manifold; a second independently-controllable exposure unit coupled to said inhalent manifold; and an exposure control system operably coupled to either or both said first independently-controllable exposure unit and said second independently-controllable exposure unit.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
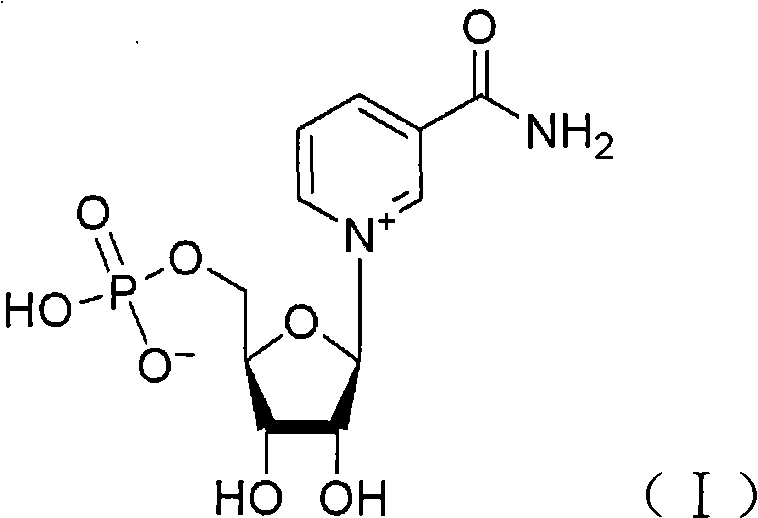
Application of nicotinamide mononucleotide
ActiveCN101601679AImprove securityEffective controlOrganic active ingredientsNervous disorderSide effectActive component
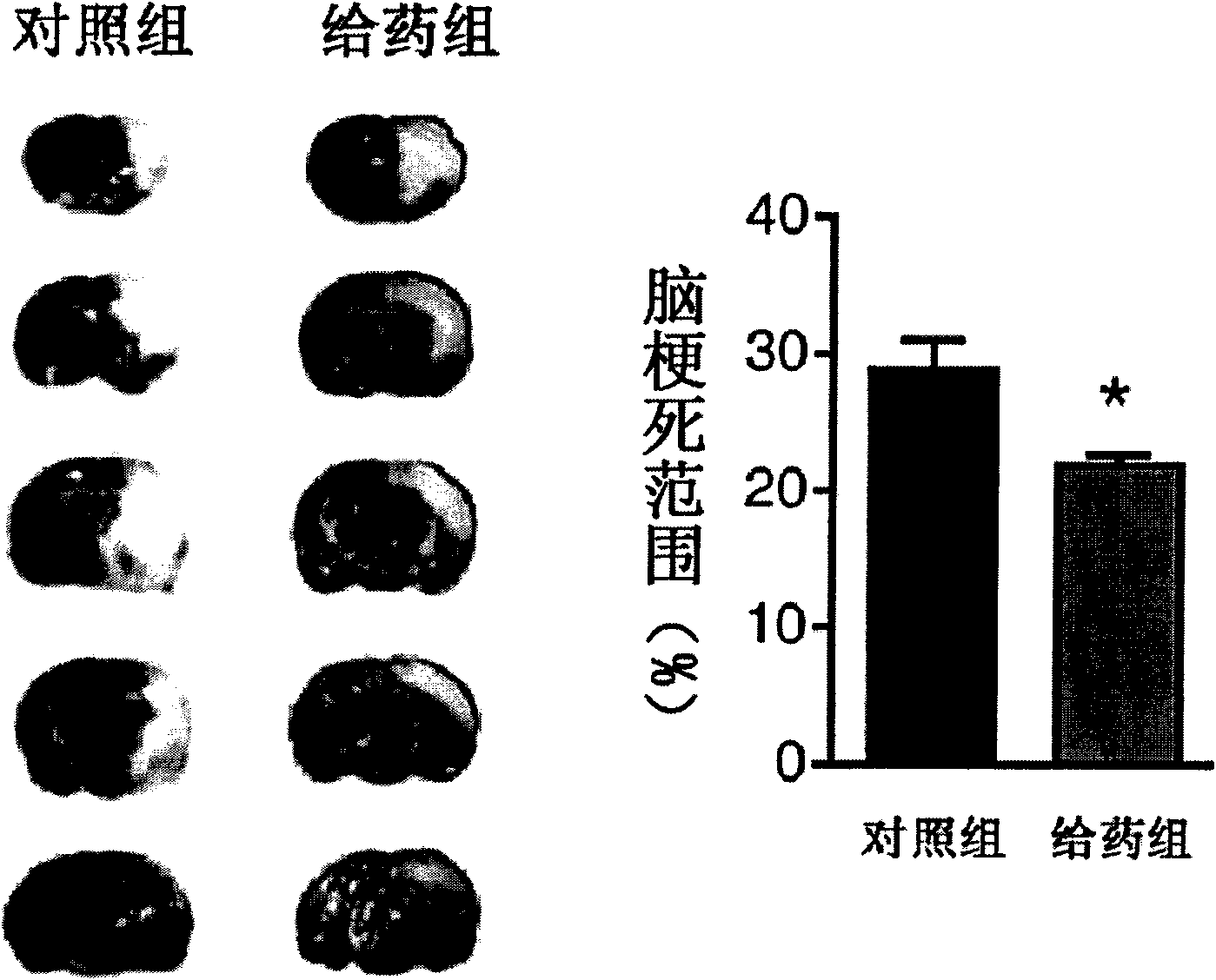
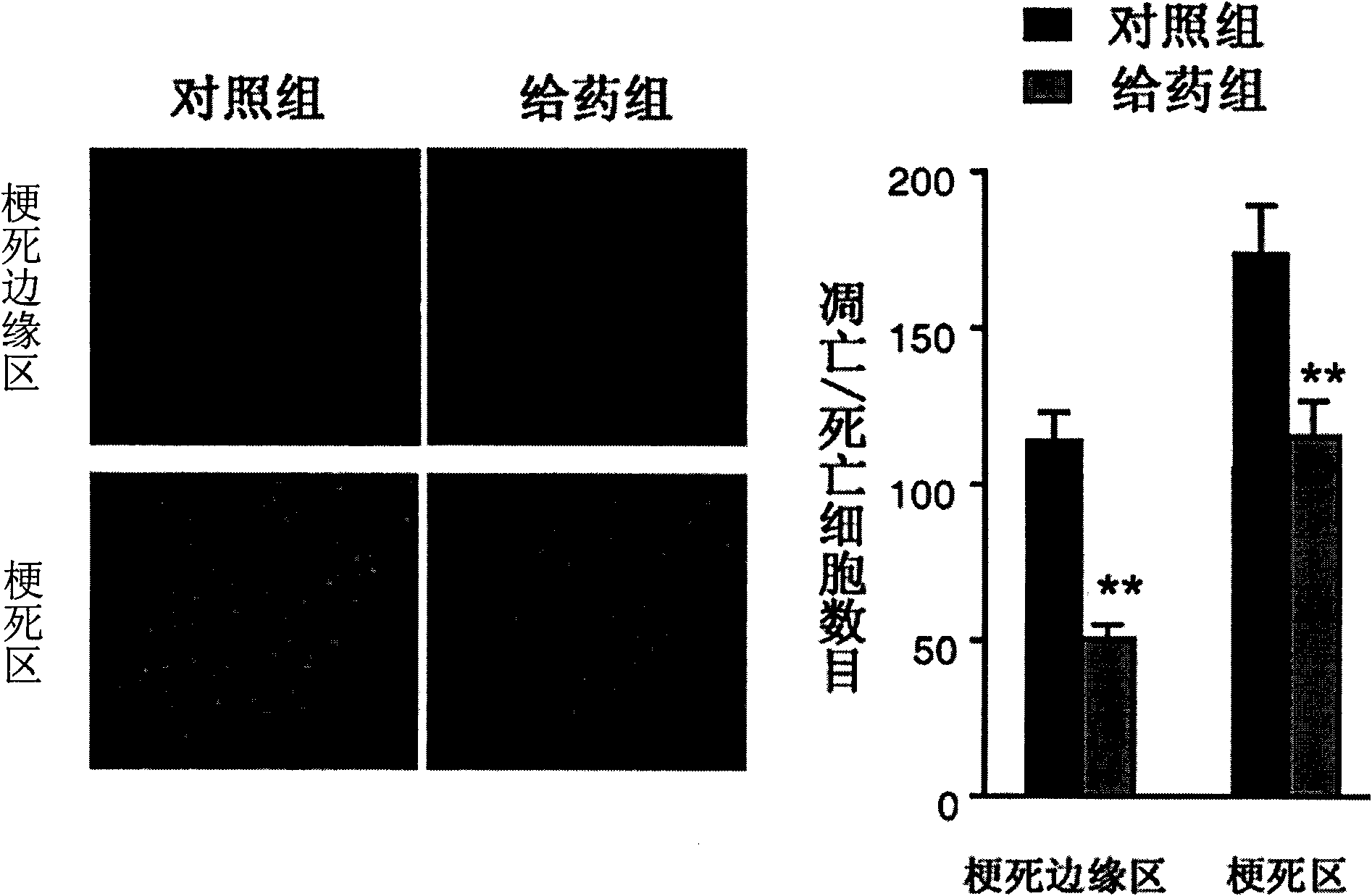
The invention discloses the application of nicotinamide mononucleotide on preparing medicines or health-care food for resisting neuronal cell injury cerebral apoplexy or oxygen-glucose deprivation. The invention also provides a novel medical composite which can effectively resist the neuronal cell injury caused by cerebral apoplexy and oxygen-glucose deprivation, thereby showing great significance to the treatment of cerebral apoplexy; and the active component in the medical composite is the nicotinamide mononucleotide which is an endogenic protective substance for an organic body and can not generate side effects according to current information, and therefore the nicotinamide mononucleotide used as a medicine has higher security and wide time window for treatment.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com