Medicine composition for treating valval and/or vaginal infection
A technology of vaginal infection and composition, applied in the field of pharmaceutical compositions, can solve the problems of large local irritation of the vagina, unsatisfactory effect, serious adverse reactions, etc., and achieves the promotion of growth recovery, significant local nutrition and anti-seborrheic dermatitis, The effect of enhancing immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: the preparation of promestriene / chlorhexidine acetate / metronidazole compound soft capsule
[0017] Weigh white petrolatum and 80% carrageenan together and heat to dissolve, keep warm at about 60°C, set aside, and make 1 liquid. Weigh chlorhexidine acetate, metronidazole and the remaining amount of carboxane together, stir to completely dissolve chlorhexidine acetate and metronidazole, then add promestriene to stir evenly, then add the weighed The sorbitan sesquioleate and ethyl paraben are stirred and dissolved, mixed with 1 solution, and fully mixed with a high-speed homogenizer to obtain the content. Add gelatin, glycerin, water, and ethylparaben according to the determined formula, melt the glue at about 60°C, and vacuum degas the glue solution for use on the capsule pressing machine. The liquid medicine and the glue liquid are introduced into the machine for preparing capsules, the machine is adjusted so that each soft capsule is injected with the liq...
Embodiment 2
[0064] Embodiment 2: Stability experiment
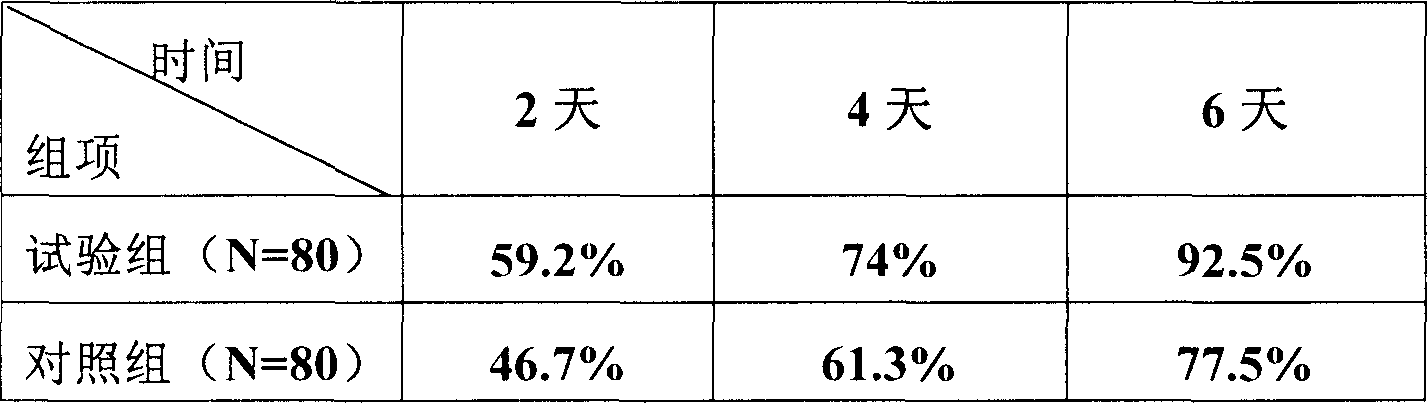
[0065] 3 batches of samples of the aluminum-plastic packaging prepared by formula 5 in Example 1 are placed at a temperature of 30 ± 2°C and a relative humidity of 60% ± 5°C (NaNO 2 Saturated solution) in the incubator, samples were taken at the end of the 1st, 2nd, 3rd, and 6th months, and tested according to the relevant stability inspection items. The results are shown in Table 1 below.
[0066]
[0067] The above stability test results show that the compound preparation has good stability, and its properties are very stable under aluminum-plastic packaging, room temperature and dry conditions.
Embodiment 3
[0068] Example 3: Local irritation and toxicity studies
[0069] Soft capsules for experiment were prepared according to formula 5 in Example 1, which contained 8 mg of promestriene, 6 mg of chlorhexidine acetate, and 160 mg of metronidazole. Each preparation contains 174 mg of medicinal ingredients, and each preparation is about 2.000 g. Female rabbits (body weight 2.20-2.60 kg) and female rats (0.225-0.270 kg) provided by the Animal Center Laboratory of West China Medical University were used as experimental animals to carry out local irritation and toxicity tests on the compound soft capsules.
[0070] Use the content of the compound soft capsule prepared above as the test drug; use compound chlorhexidine as the positive control drug, and the administration and dosage are equivalent to 6 mg of chlorhexidine acetate and 160 mg of metronidazole; Soft capsules were used as negative control.
[0071] In the experiment, the test drug was divided into the following three doses:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com