Medicine composition for treating valval and/or vaginal diseases
A technique for vaginal diseases and compositions, applied in the field of pharmaceutical compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
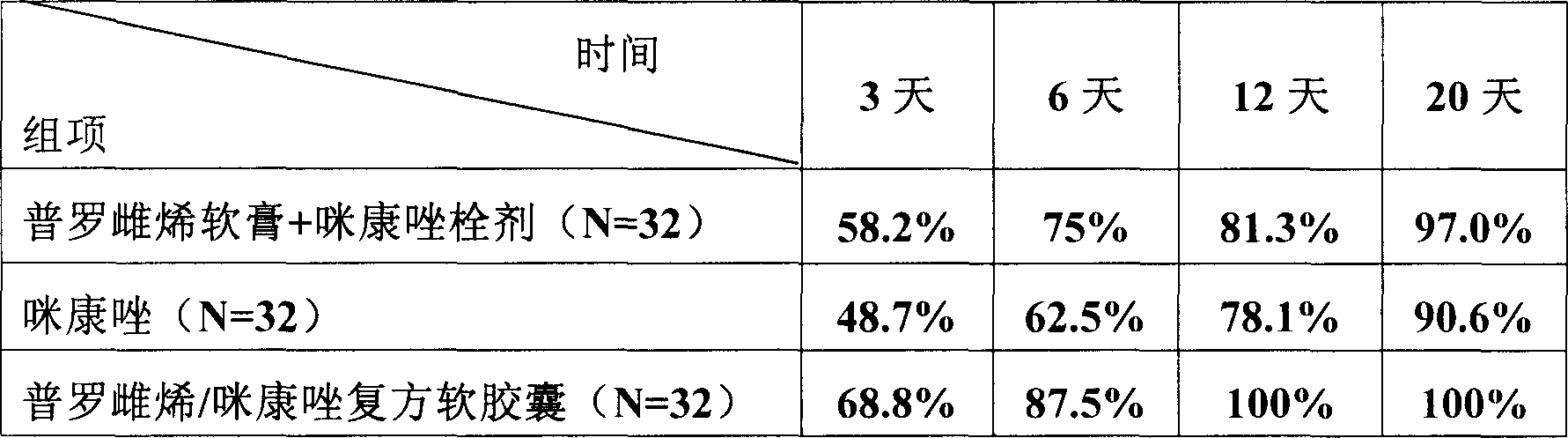
Embodiment 1
[0019] According to following formula, make the soft capsule that meets quality standard with the production technology of capsule:
[0020] Recipe 1
[0021] Component Content (mg / grain)
[0022] Promestriene 10
[0023] Miconazole 50
[0024] White Vaseline 500
[0025] Carrageenan 900
[0026] Ethylparaben 200
[0027] Sorbitan Sesquioleate Added to 2000mg, made into 1000 capsules
[0028] Recipe 2
[0029] Component Content (mg / grain)
[0030] Promestriene 8
[0031] Miconazole 60
[0032] White Vaseline 500
[0033] Carrageenan 900
[0034] Ethylparaben 200
[0035] Sorbitan Sesquioleate Added to 2000mg, made into 1000 capsules
[0036] Recipe 3
[0037] Component Content (mg / grain)
[0038] Promestriene 8
[0039] Miconazole 55
[0040] White Vaseline 400
[0041] Carrageenan 900
[0042] Ethylparaben 200
[0043] Sorbitan Sesquioleate Added to 2000mg, made into 1000 capsules
[0044] Recipe 4
[0045] Component Content (mg / grain)
[0046] Promestr...
Embodiment 2
[0065] Embodiment 2: local irritation and acute toxicity research,
[0066] Tested drug: Promestriene / miconazole compound soft capsules, each capsule content (2g) contains 7mg promestriene, miconazole 50mg soft capsules, each gram content contains 0.0285g of drug ingredients.
[0067] High dose: 1.0g per day for each rabbit, 0.5g for one dose, twice a day, 0.0285g of drug ingredients, with an average of 11.9mg / kg, which is 20 times the dosage for humans. Each rat is given 0.2g once a day, and once a day (about 0.2g) the drug composition is 5.7mg, with an average of 23mg / kg, which is 40 times the dosage for humans.
[0068] Medium dose: each rabbit is given 0.5g per day, 0.25g once a day, twice a day, the drug component is 0.0143g, with an average of 5.95mg / kg, which is 9.5 times the dosage for humans. Each rat is given 0.1g once a day, and once a day (about 0.1g) the drug composition is 2.85mg, with an average of 11.5mg / kg, which is 20 times the dosage for humans.
[0069] L...
Embodiment 3
[0075] Example 3: Long-term Toxicity Study of Topical Drugs
[0076] Test drug: same as above
[0077] High dose: each rat is given 0.2g once, once a day (about 0.2g) the drug ingredient is 5.7mg, with an average of 23mg / kg, which is 40% of the human dosage.
[0078] Medium dose: each rat is given 0.1g once a day, once a day (about 0.1g) the drug ingredient is 2.85mg, with an average of 11.5mg / kg, which is 20 times the dosage for humans
[0079] Low dose: 0.05g administered once per rat, 1.43mg of drug ingredient once a day (about 0.05g), with an average of 5.75mg / kg, which is 10 times the dosage for humans
[0080] The body weight of female rats is 0.235-0.260kg. The three doses were administered once a day, continuously observed for 30 days, and the test was repeated three times. The results showed that no toxic reaction and local irritation were found in the animal's whole body and vagina; Drugs in the dose group had no pathogenic effect on each layer of the vagina.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com