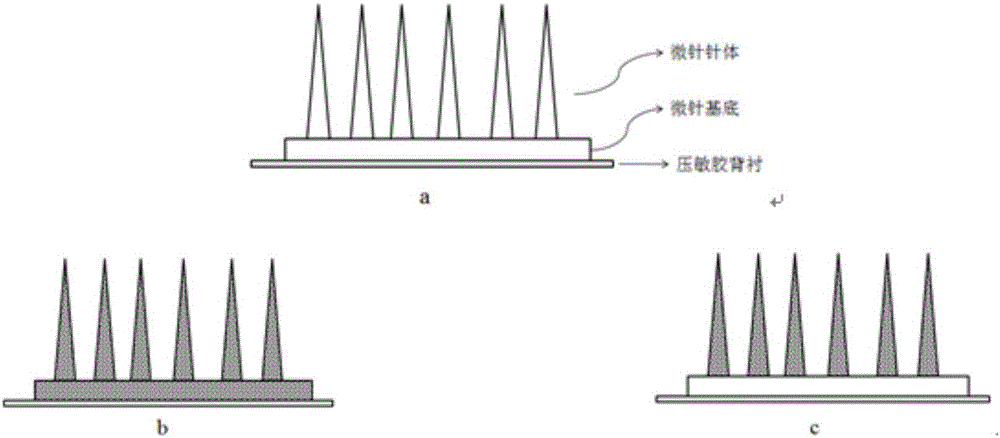
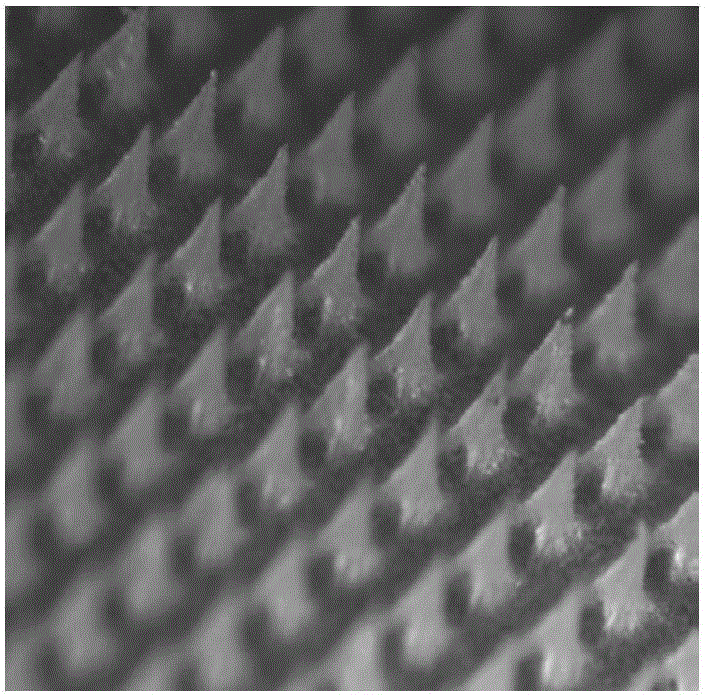
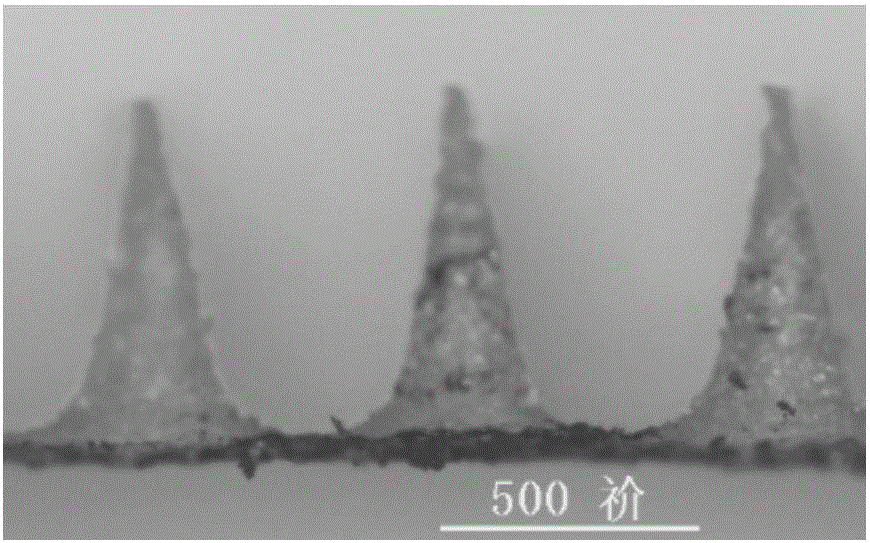
Flexible slow-release micro-needle patch and preparation method thereof
A micro-needle sticking, flexible technology, applied in the field of medicine, can solve the problems of inability to meet the production of large-dose pharmaceutical preparations, low drug loading of micro-needles, organic solvent residues, etc., and achieves easy control of solubility, simple process, and conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Preparation of sustained-release microneedle patch for intradermal administration
[0044] 1) Weigh 0.5 g of carboxymethyl cellulose with a molecular weight of 200 KDa, add 4.5 ml of water, and prepare a carboxymethyl cellulose aqueous solution with a mass fraction of 10%.
[0045] 2) Grinding the raw material medicine of paclitaxel in crystal form and sieving to obtain drug crystals with a particle size of about 10 μm. Weigh 100 mg of the sieved drug crystal and add it to the above-mentioned carboxymethyl cellulose aqueous solution, and magnetically stir (1500rpm, 12h) to obtain a microneedle emulsion (ie, microneedle injection molding solution) with a mass ratio of matrix material to drug of 5:1. .
[0046] 3) Add 80 μl of microneedle emulsion into the microneedle mold, and make the microneedle emulsion enter the microneedle pinholes by vacuuming.
[0047] 4) Dry the above-mentioned microneedle mold with the microneedle emulsion added under the conditions...
Embodiment 2
[0051] Example 2: Preparation of sustained-release microneedle patch for intradermal administration
[0052] 1) Weigh 2 g of PVP K30, add 4 ml of water, and prepare an aqueous solution of polyvinylpyrrolidone with a mass fraction of 33%.
[0053] 2) Grinding and sieving the bulk drug in the crystal form of etonogestrel to obtain drug crystals with a particle size of about 15 μm. Weigh 100 mg of sieved drug crystals and add them to the aqueous solution of PVP K30, and magnetically stir (2000 rpm, 6 h) to prepare a microneedle emulsion (microneedle injection molding solution) with a mass ratio of matrix material to drug of 20:1.
[0054] 3) Add 150 μl of microneedle emulsion into the microneedle mold, and pressurize the microneedle emulsion into the microneedle pinholes. Microneedle patch 100 needles / square centimeter, needle length 0.50mm.
[0055] 4) Dry the above-mentioned microneedle mold with the microneedle emulsion added under the conditions of 25° C. and 30% humidity f...
Embodiment 3
[0059] Example 3: Preparation of sustained-release microneedle patch for intradermal administration
[0060] 1) Weigh 0.5 g of sodium hyaluronate with a molecular weight of 1500 KDa, add 9.5 ml of water, and prepare an aqueous solution of sodium hyaluronate with a mass fraction of 5%.
[0061] 2) Grinding the raw material drug of artemether in crystal form and then sieving to obtain the drug in crystal form with a particle size of 20 μm. Weighed 50 mg of the sieved drug in crystal form and added it to the aqueous solution of hyaluronic acid, and stirred it magnetically (1000 rpm, 12 h) to prepare a microneedle emulsion with a mass ratio of matrix material to drug of 10:1.
[0062] 3) Add 100 μl of microneedle emulsion into the microneedle mold, and make the microneedle emulsion enter the microneedle pinholes by vacuuming. The microneedle patch has 169 needles / square centimeter, and the needle length is 0.85mm.
[0063] 4) Put the above-mentioned microneedle mold added with t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com