Preparation method of pentafluoroethane
A technology of pentafluoroethane and tetrafluoroethane, which is applied in the field of preparation of pentafluoroethane, can solve the problems of unstable material ratio in fluorination reactor, different catalysts, complex process and actual operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
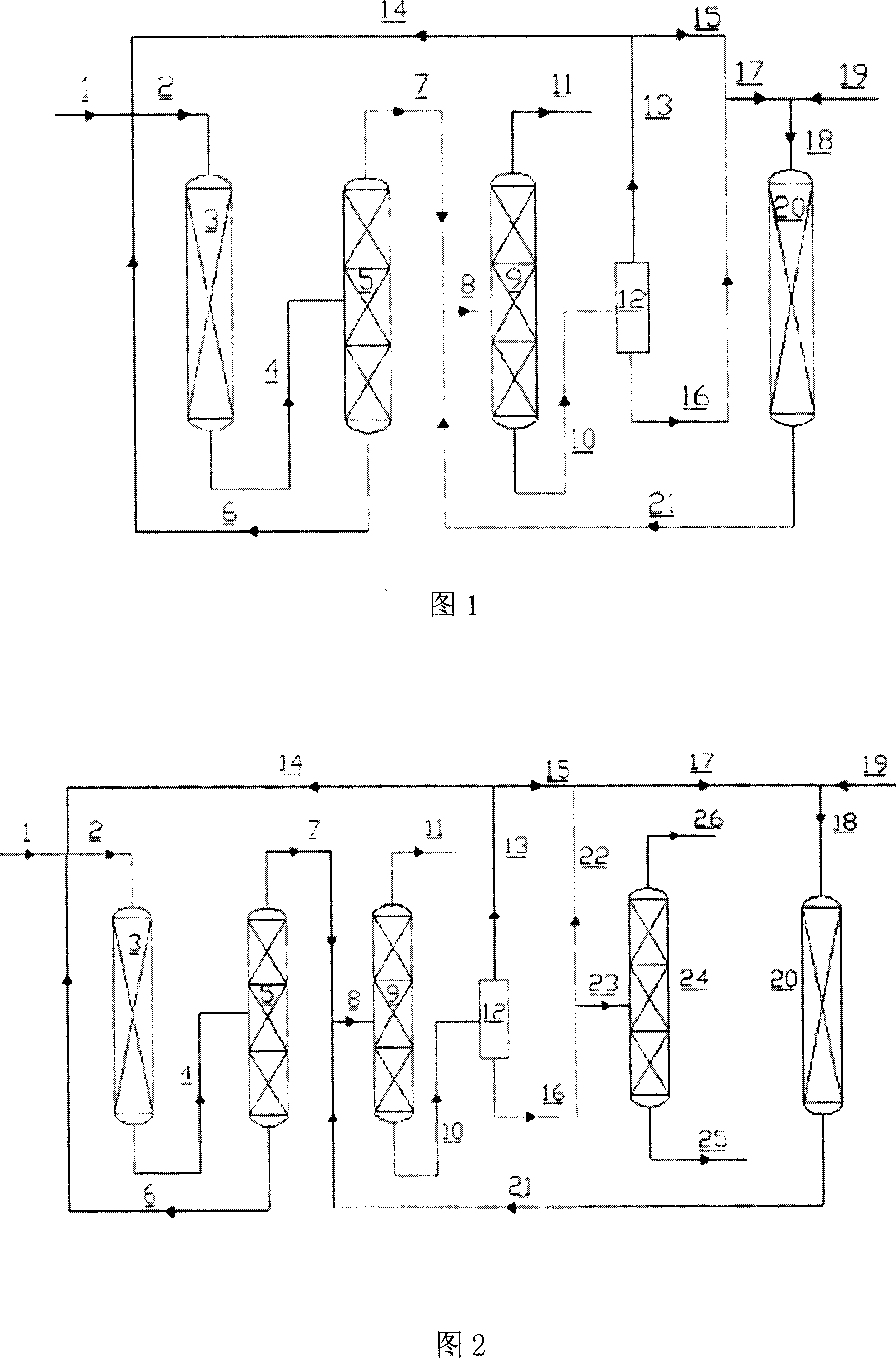
[0029] The present invention is further described in detail with reference to FIG. 1 . The fresh PCE or the mixture of PCE and HF enters the first reactor 3 filled with fluorination catalyst through the pipeline 1 together with the recycled HF stream and the recycled PCE stream. The reaction is carried out, and the reaction product flows through the pipeline 4 and enters the distillation tower 5 for separation. The PCE containing a small amount of HF in the tower bottom component is circulated into the first reactor through the pipeline 6, and the top components HCFC-123, HCFC-124, HFC-125 , HF and HCl enter the distillation column 9 through pipelines 7 and 8 for separation. The top components of the distillation tower 9 are HFC-125 and HCl, which enter the product post-treatment system through the pipeline 11, and the HFC-125 product can be obtained through acid removal, dehydration, and rectification; the still components enter the phase separator 12 through the pipeline 10 ...
Embodiment 2
[0034] The present invention is further described in detail with reference to FIG. 2 . Figure 2 shows the pentafluoroethane preparation method for the co-production of HCFC-123 and HCHC-124. On the basis of the pentafluoroethane production method shown in Figure 1, a distillation tower for separating HCFC-123 and HCFC-124 is added twenty four. In this case, the lower layer of the phase separator 12 is an organic phase rich in HCFC-123 and HCFC-124, a part of which is recycled to the second reactor 20 through lines 16, 22 for reaction, and another part of which is passed through lines 16, 22 Enter the distillation tower 24 for separation, the bottom component is HCFC-123, and the top component is HCFC-124. The separated HCFC-123 and HCFC-124 enter the product post-treatment system through pipelines 25 and 26 respectively, and the final products are obtained after deacidification, dehydration and rectification.
[0035] The main logistics composition in the embodiment is shown...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com