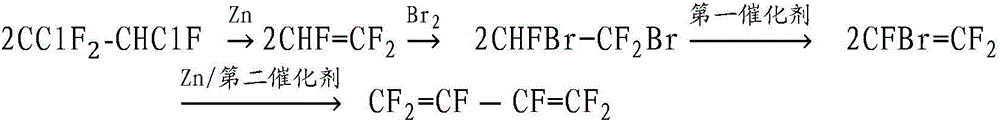Method for preparing hexafluoro-1,3-butadiene
A technology for catalytic preparation of -butadiene, which is applied in the direction of dehalogenation preparation, dehydrohalogenation preparation, chemical instruments and methods, etc., can solve the problems of harsh reaction conditions, high raw material prices, and difficult handling of reagents, and achieve low cost, The effect of high yield and few steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] (2) The method for preparing hexafluoro-1,3-butadiene provided by the present invention overcomes the disadvantages of long operation period and low yield per unit volume of the existing process, and has high yield, few steps and easy industrialization. Through the selection of reactants and the control of reaction conditions, the production of high-purity hexafluoro-1,3-butadiene can be realized.
[0041] Further, in step 1, in terms of mass ratio, solvent: zinc powder=1.5~4:1; in terms of molar ratio, zinc powder: 1,2-dichloro-1,1,2-trifluoroethane= 1 to 2:1, more preferably 1 to 1.5:1.
[0042] Further, the solvent is selected from one of tetrahydrofuran, acetonitrile and N,N-dimethylformamide.
[0043] Further, the zinc powder was pretreated with 10wt% hydrochloric acid solution for 20 minutes before being loaded into the reactor, washed with absolute ethanol, and then dried at 100°C.
[0044] Further, in step 1, the reaction temperature is 60-80° C., and the reac...
Embodiment 1
[0057] Example 1 Preparation of CHF=CF from 1,2-dichloro-1,1,2-trifluoroethane 2 and CBrF 2 CHBrF
[0058] Using 1,2-dichloro-1,1,2-trifluoroethane as raw material, CHF=CF was prepared by liquid-phase dechlorination reaction 2 . 200g of zinc powder was pretreated with 10% hydrochloric acid solution for 20min, washed with absolute ethanol, and dried at 100°C. Put the treated Zn into a stainless steel reactor with stirring, and feed N 2 Purge for 0.5h. Add 400g of tetrahydrofuran solvent into the reaction kettle, and start stirring. Then gradually feed 600g of 1,2-dichloro-1,1,2-trifluoroethane under stirring, raise the temperature of the reactor to 70°C under stirring, react for 1 hour, and take samples after the reactor is naturally cooled Analysis, the conversion rate of 1,2-dichloro-1,1,2-trifluoroethane is 94.3%, CHF=CF 2 The selectivity is 98.2%. The gas produced by the reaction is washed with water, washed with alkali, and passed into liquid bromine. After the rea...
Embodiment 2
[0059] Example 2 Preparation of CHF=CF from 1,2-dichloro-1,1,2-trifluoroethane 2 and CBrF 2 CHBrF
[0060] Using 1,2-dichloro-1,1,2-trifluoroethane as raw material, CHF=CF was prepared by liquid-phase dechlorination reaction 2 . 300g of zinc powder is put into a stainless steel reaction kettle with stirring, and N 2 Purge for 0.5h. 500g of acetonitrile solvent was added to the reaction kettle, and the stirring was started. Then gradually feed 600g of 1,2-dichloro-1,1,2-trifluoroethane under stirring, raise the temperature of the reactor to 80°C under stirring, react for 2 hours, and take samples after the reactor cools naturally Analysis, the conversion rate of 1,2-dichloro-1,1,2-trifluoroethane is 96.5%, CHF=CF 2 The selectivity is 97.8%. The gas produced by the reaction is washed with water, washed with alkali, and passed into liquid bromine. After the reaction is complete, the color of liquid bromine gradually becomes lighter from brown, CHF=CF 2 and Br 2 Complete ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com