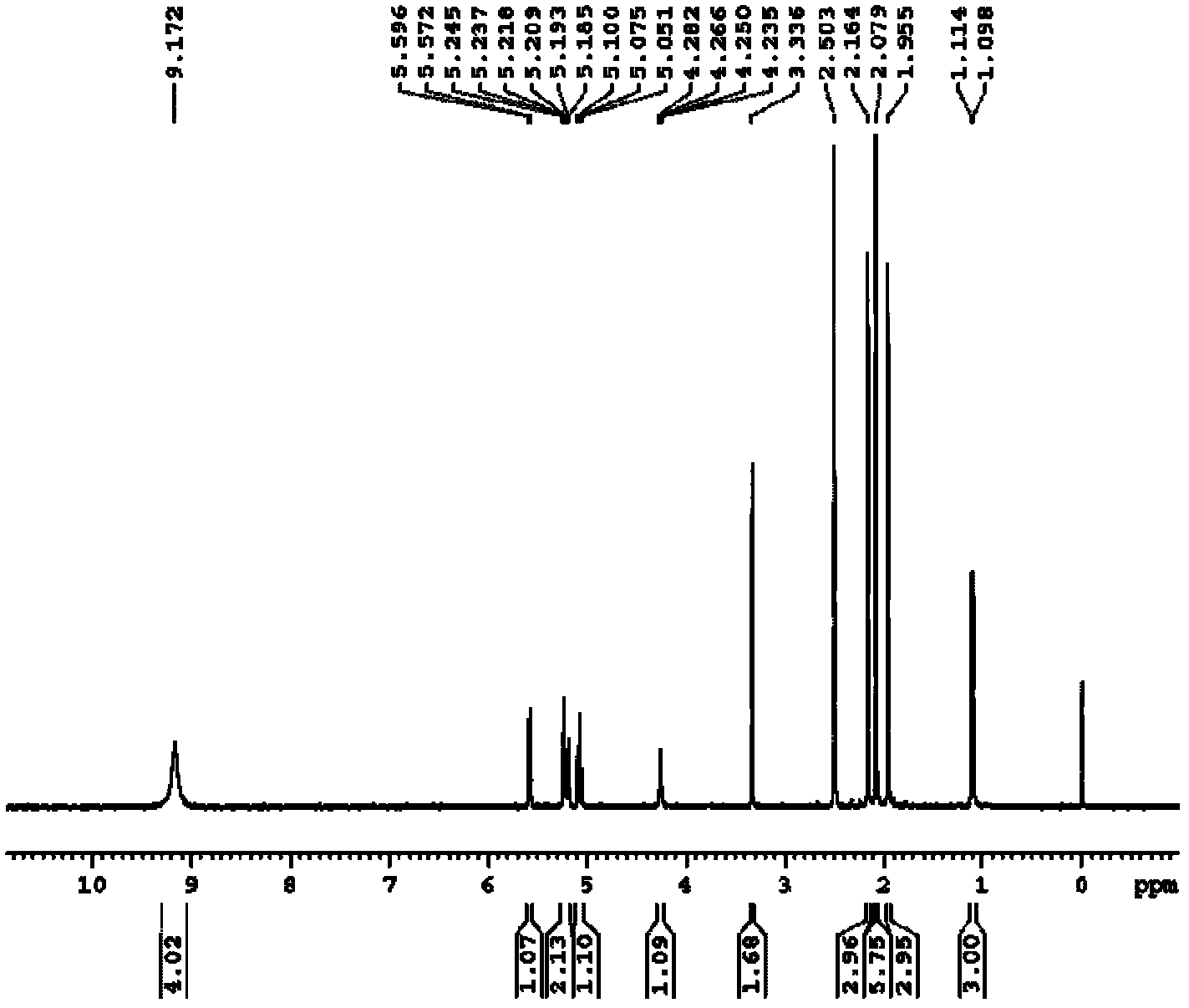
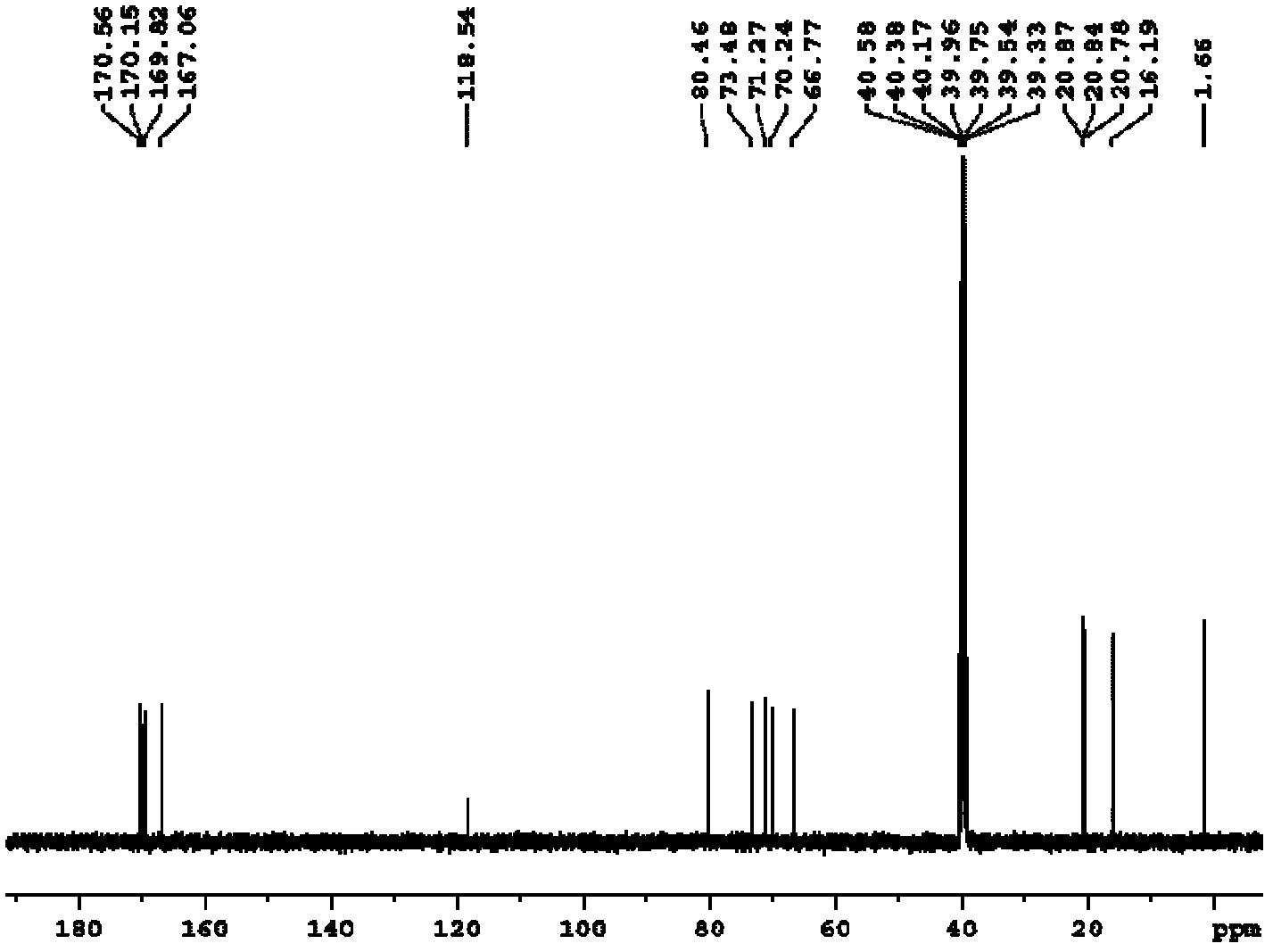
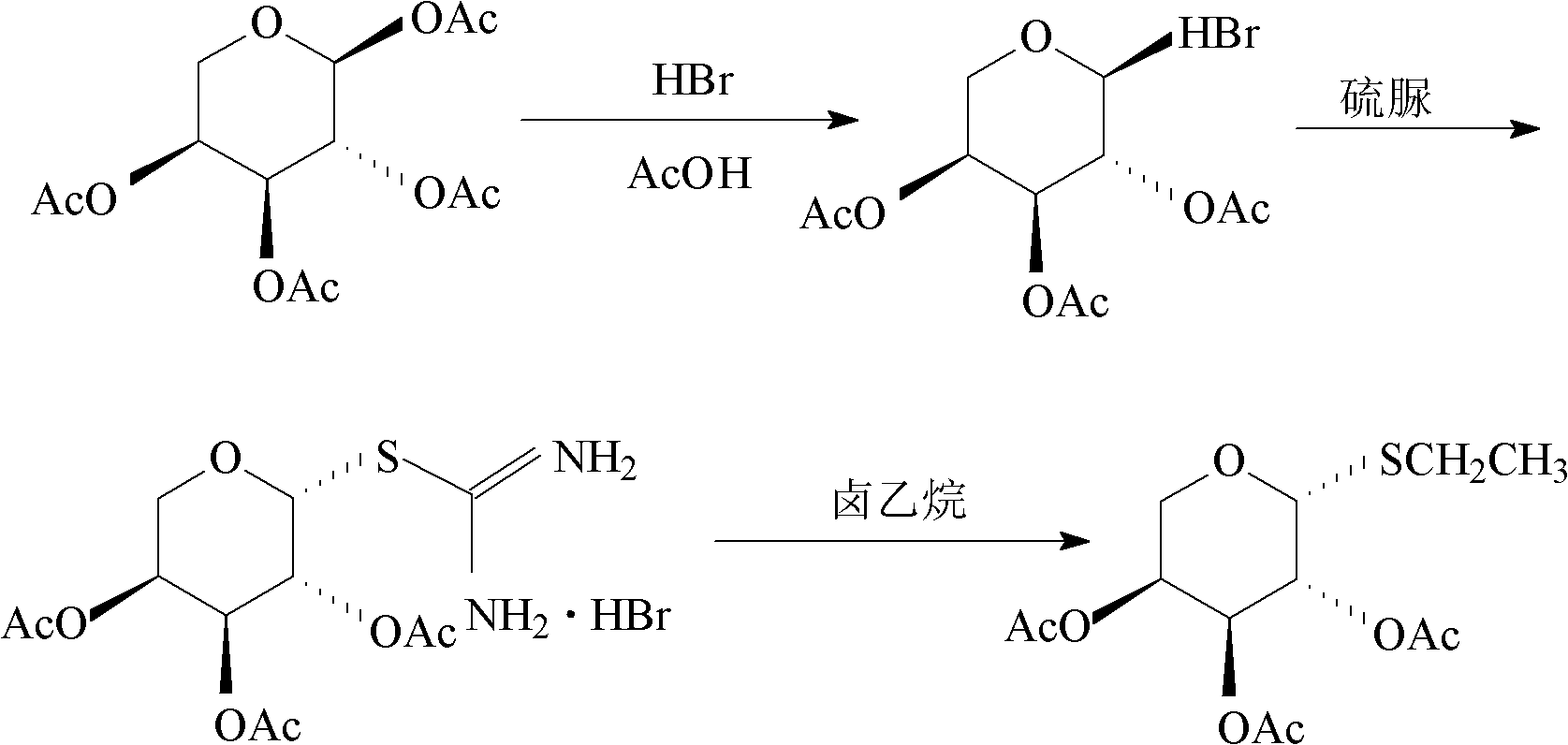
Preparation method of thioglycoside compound
A technology of thioglycosides and compounds, which is applied in the field of preparation of thioglycoside compounds, can solve the problems of complex treatment of thioglycoside compounds and cumbersome operation steps, and achieve the goal of avoiding harm to human health and the environment and simple post-processing work Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The embodiment of the present invention discloses a preparation method of a thioglycoside compound, comprising the following steps:
[0025] a) dissolving the haloacetylated sugar compound and thiourea in the first organic solvent, and reacting under heating conditions to obtain the halogenated S-glycosylisothiourea;
[0026] b) The S-glycosylisothiourea halide reacts with haloethane in a mixed solution of a second organic solvent and an organic amine to obtain a thioglycoside compound.
[0027] Step a) is the reaction of the haloacetylated sugar compound with thiourea to obtain the intermediate product S-glycosylisothiourea halide. The above-mentioned first organic solvent is used as a reaction medium, and here only plays the role of a solvent, and it does not react with the raw materials as a reaction medium. The above-mentioned first organic solvent is preferably acetonitrile, dichloromethane or ethyl acetate, more preferably acetonitrile. The above reaction can on...
Embodiment 1
[0032] a) Add 162ml of 33wt% acetic acid / HBr solution to 160g tetraacetyl-L-arabinose, stir to make it dissolve, react for about 1.5h, test the reaction process by TLC spot plate; the reaction is considered complete when the raw material point disappears; Add 500ml dichloromethane in the reaction solution, add the Na of 5wt% again 2 CO 3 200ml of the solution was used to neutralize the pH value to 7, and the organic phase was separated by stirring. The organic phase was dried with anhydrous sodium sulfate and evaporated to dryness at 40°C to obtain bromotetraacetyl-L-arabinose with a yield of 639g;
[0033] b) Add the above 165g of bromotetraacetyl-L-arabinose to 630ml of acetonitrile to dissolve it, and heat it to 60°C; slowly add 36.5g of thiourea to the reaction solution, when the reflux starts, white flocs are formed ; After adding thiourea, react for about 30 minutes and test the reaction process with TLC spot plate until the raw material spots disappear; after the react...
Embodiment 2
[0036] a) Add 478ml of 33wt% acetic acid / HBr solution to 450g tetraacetyl-L-arabinose, stir to make it dissolve, react for about 1.5h, test the reaction process by TLC spot plate; the reaction is considered complete when the raw material point disappears; then Add 1.5L dichloromethane in the reaction solution, add the Na of 5wt% again 2 CO 3 600ml of the solution was used to neutralize the pH value to 7, and the organic phase was separated by stirring. The organic phase was dried with anhydrous sodium sulfate and evaporated to dryness at 40°C to obtain bromotetraacetyl-L-arabinose with a theoretical yield of 480g.
[0037]b) Add 480g of the above-mentioned bromoacetylated sugar compound into 1.86L of acetonitrile to dissolve it, and heat it to 75°C; slowly add 107.6g of thiourea to the reaction solution, and white flocs will form when the reflux begins; After adding urea, react for about 30 minutes and test the reaction progress with TLC spot plate until the raw material spot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com