Method for measuring concentration of volatile anesthetics in samples by chromatography
A measurement method and volatility technology, applied in the field of biomedicine, can solve the problems of long measurement time and inconvenient storage, and achieve the effects of accurate results, reduced pollution, and shortened sample detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The present invention will be further described in conjunction with specific examples, but not limited thereto. The mensuration of volatile anesthetic concentration in blood of embodiment 1
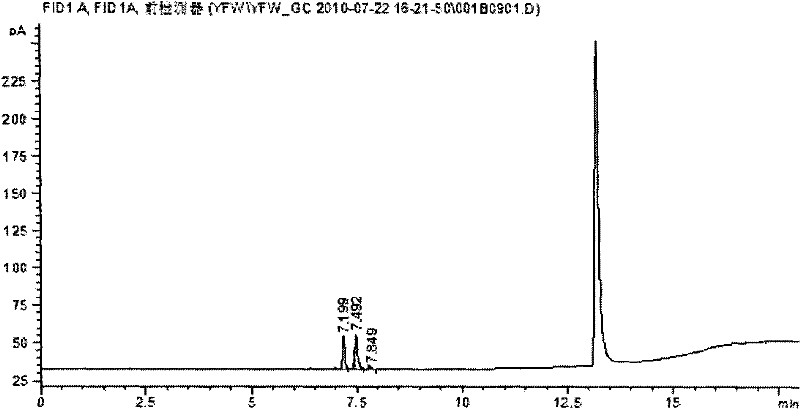
[0039] (1) Chromatographic conditions Agilent6890N gas chromatograph, DB-WAX capillary chromatographic column, inlet temperature 160°C, carrier gas high-purity nitrogen, column flow rate 2ml / min. The temperature is programmed to rise, starting at 60°C for 2 minutes, rising to 200°C at a speed of 10°C / min, maintaining for 2 minutes, and running for 18 minutes in total. The detector is a flame ionization detector (FID), and the detector temperature is 300°C. Automatic headspace sampling, heat preservation at 85°C for 30min, quantitative loop temperature at 90°C, transfer line temperature at 120°C, injection volume 1ml. (same chromatographic conditions)
[0040] (2) Preparation of standard stock solution Take an appropriate amount of sevoflurane reference substance, dissolve and di...
Embodiment 2
[0046] Example 2 The isoflurane concentration of the same whole blood sample was determined by automatic headspace sampling method and syringe headspace secondary balance method.
[0047] Experimental operation
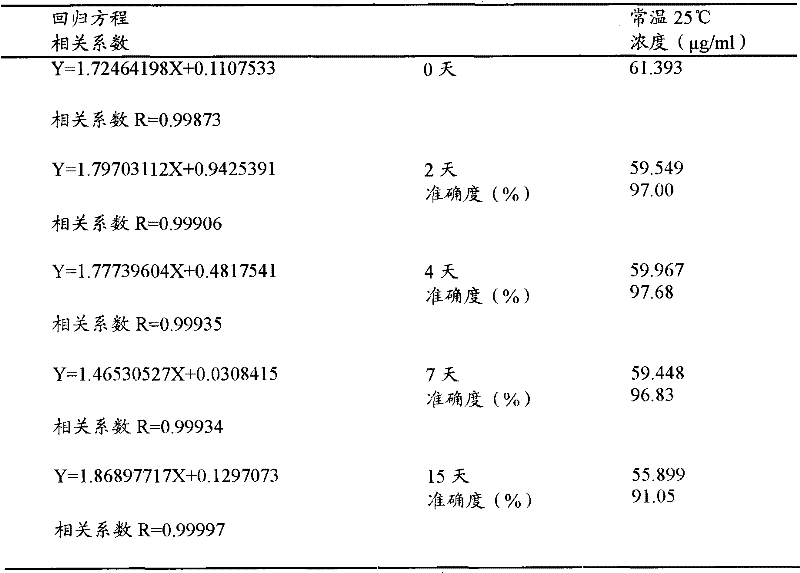
[0048]Take two blank whole blood samples of 20ml each, add a certain amount of isoflurane reference substance respectively, and wait for one day to equilibrate at room temperature 25°C to obtain blood samples 1 and 2 containing isoflurane, and draw blood samples 1 and 2 using the secondary balance method of a syringe , to determine the concentration of isoflurane; another blood sample 1, 2 each 1ml was added to the headspace bottle and sealed with a cap, and this method was used to determine the concentration of isoflurane.
[0049] Experimental results
[0050] Table 3 Determination of isoflurane concentration in the same whole blood sample by automatic headspace sampling method and syringe headspace secondary equilibrium method
[0051]
[0052] (Accuracy is th...
Embodiment 3
[0054] The mensuration of volatile anesthetic concentration in embodiment 3DMF aqueous solution
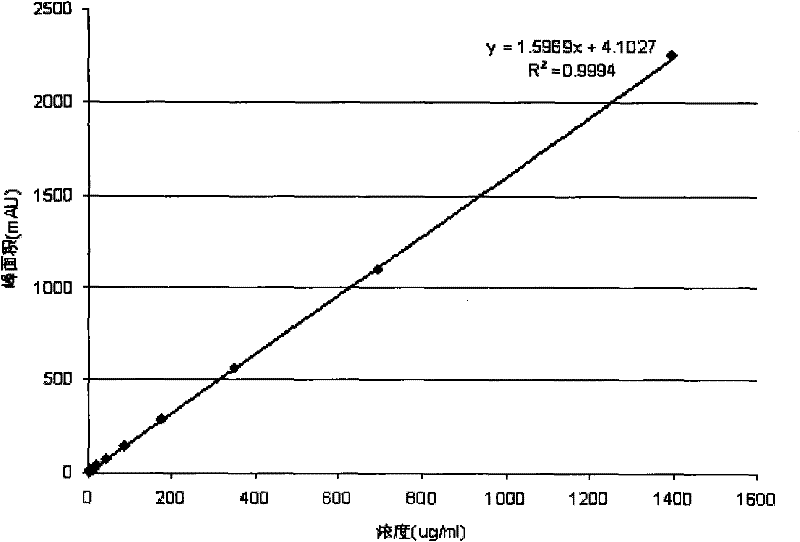
[0055] Take an appropriate amount of volatile anesthetic, weigh it accurately, add DMF aqueous solution to dissolve and dilute to make a stock solution, and use the stock solution to double-dilute to 6-10 solutions of different concentrations as the solution to be tested. Take 0.5-1ml respectively and put them in the headspace bottle. Seal the bottle; measure the peak areas of samples with different concentrations under the chromatographic conditions of Example 1 for comparison. Use the linear regression of the peak area (Y-axis) of the volatile anesthetic isoflurane to the concentration (X-axis), draw a standard curve, and obtain the regression equation
[0056] Y=1.5969X+4.1027 R2=0.9994 The results show that the linearity is good, which is in line with the "Technical Guidelines for Clinical Pharmacokinetic Research of Chemical Drugs". See figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com