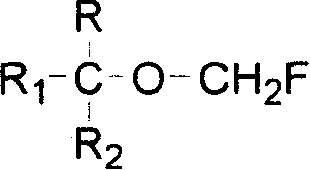
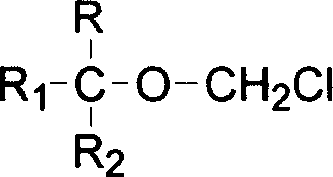
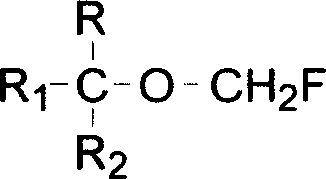Process for preparing fluoro methyl ether
A monochloromethyl ether, fluorine technology, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry and other directions, can solve problems such as low reaction conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 500g of chloromethyl ether, 100g of sodium fluoride and 45g of ethylene glycol into a 500mL single-necked bottle with a magnetic stirring and reflux device, heat and reflux for 5 hours, and after the reaction is completed, 432.2g is obtained by distillation, which contains heptafluoride after analysis Alkanes 96.5%, yield: 90.3%.
Embodiment 2
[0026] Add 500g of chloromethyl ether, 140g of potassium fluoride, 400mL of sulfolane and 60g of 18-crown ether-6 into a 1000mL single-necked bottle equipped with a magnetic stirring and reflux device, heat and reflux for 2 hours, and after the reaction is completed, 419.6g of the product is obtained by distillation After analysis, it contains 95.3% of sevoflurane, and the yield is 86.6%.
Embodiment 3
[0028] Add 500g of chloromethyl ether, 80g of potassium fluoride and 30g of 18-crown-6 into a 500mL single-necked bottle equipped with a magnetic stirring and reflux device, heat and reflux for 4 hours, and after the reaction is completed, 236.6g of the product is obtained by distillation and recovered 204.2g of chloromethyl ether, the product contains 98.8% of sevoflurane after analysis, yield: 84.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com