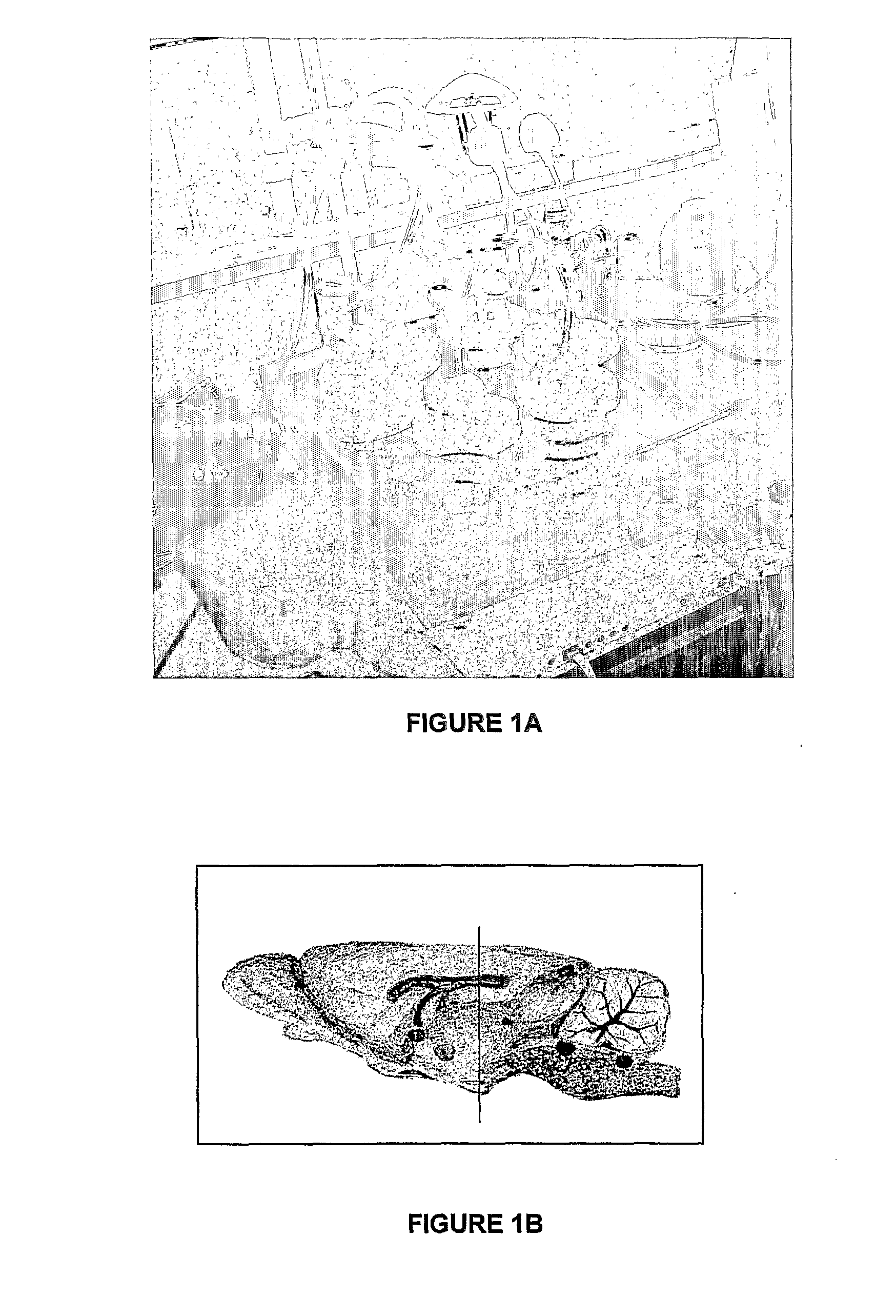
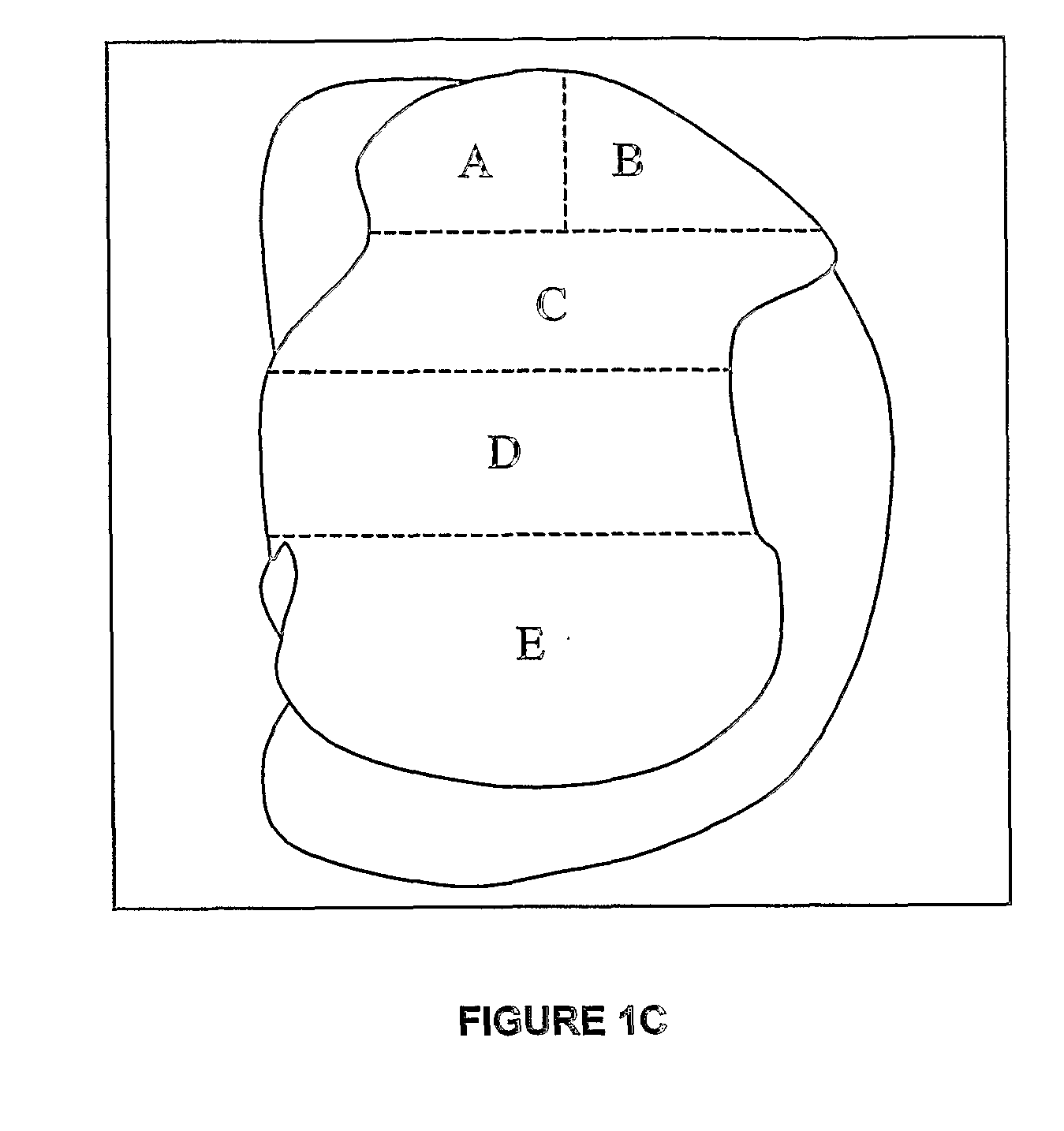
Use of xenon as neuroprotectant in a neonatal subject
a technology of neuroprotectant and xenon, which is applied in the direction of antinoxious agents, drug compositions, biocides, etc., can solve the problems of significant cost involved in the production of xenon through fractional distillation of liquid air, failure to materialize every day use of xenon anesthesia, and relatively small percentage of the total refined quantity of xenon available for anesthesia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Exposure to Anesthetic Gases
Animals
[0200]7 day old Sprague-Dawley rat pups were placed in individual wells of a custom-built anesthetic chamber, and randomised to groups A-F to receive one of 6 gas combinations for 6 hours. Previous work has established that NMDA receptor antagonists have their maximal neurodegenerative affect 7 days after birth (Ikonomidou et al., 1999).
Gas Delivery
[0201]Group B received 75% nitrous oxide and 25% oxygen as delivered by a calibrated anesthetic machine, whereas group C received 75% xenon along with 25% oxygen through a customised anesthetic machine modified for xenon delivery (Ohmeda, modified by Air Products, Surrey, UK). Group D were exposed to 25% oxygen along with 0.75%; isoflurane. The remaining 2 groups were exposed to combinations of gases—namely 25% oxygen+75% A nitrous oxide+0.75% isoflurane (group E) and 25% oxygen+60% xenon+15% nitrogen+0.75% isoflurane (group F). The high cost of xenon precludes its use in an open-circuit, consequently gr...
example 2
Methods
Neuronal Glial Co-Culture
[0217]Whole cerebral neocortices (devoid of the hippocampus, basal ganglia and meninges) were obtained from early post natal (1-2 day old) pups of BALB / c mice. The pups were anaesthetised with isoflurane and then decapitated with the heads placed immediately into 4° C. HSG solution, an isotonic, high sucrose glucose solution made primarily from Hank's balanced salt solution (HBSS, GibroBRL) enhanced with NaHCO3 (0.04 M), sucrose (0.2 M) and D-Glucose (0.3 M) also containing antibiotic-anti-mycotic solution (AAS, GibroBRL). Throughout the micro dissection process, brain tissues were kept in 4° C. HSG solution.
[0218]The brain tissue was then immersed in 0.25% trypsin and a placed in a shaking air chamber for 50 minutes at 37° C. filled with 5% CO2 and 95% room air. DNase was then added to the mixture and placed back into the shaking air chamber for a further 15 minutes. The mixture was then centrifuged at 1600 rpm for 10 minutes at 4° C. and the superna...
PUM
| Property | Measurement | Unit |
|---|---|---|
| blood/gas partition coefficient | aaaaa | aaaaa |
| v/v | aaaaa | aaaaa |
| v/v | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com