Use of formulations containing halogenated volatile anesthetics for the preparation of formulations for the treatment of patients whose tissues have ischemic events by parenteral administration
A technology of volatile, anesthetics, applied in the field of application of preparations containing halogenated volatile anesthetics in the preparation of preparations for the treatment of patients with ischemic events in tissues by parenteral administration, capable of solving unawareness, Unaware of issues such as tissue protection of parenteral preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1: the preparation of intravenous HVA preparation
[0064] In this example, the preparation of a sevoflurane emulsion formulation is specifically described. A similar approach was used for formulations containing enflurane, isoflurane and desflurane (see below).
[0065] Prepare the oil phase:
[0066] The container and its stirring device are tared together.
[0067] Refined soybean oil is added to it.
[0068] Heat the oil to approximately 50±5°C.
[0069] Add lecithin to the oil phase container.
[0070] Mix the contents until the lecithin is completely dissolved.
[0071] The temperature of the oil / lecithin is adjusted to about 22±2°C and maintained at this temperature.
[0072] Add the required amount of sevoflurane and maintain the temperature at 22±2°C.
[0073] Prepare the water phase
[0074] The vessel used to prepare the aqueous phase and its stirring device are tared together.
[0075] Add required water for injection to the water phase...
Embodiment 2
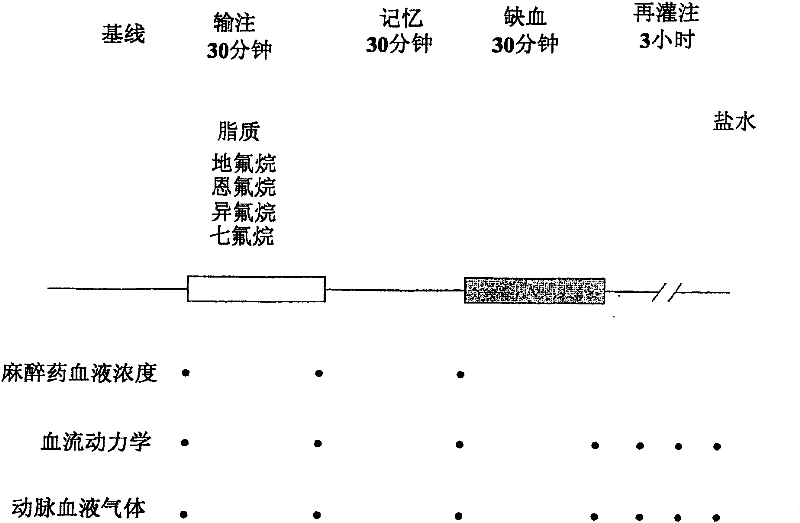
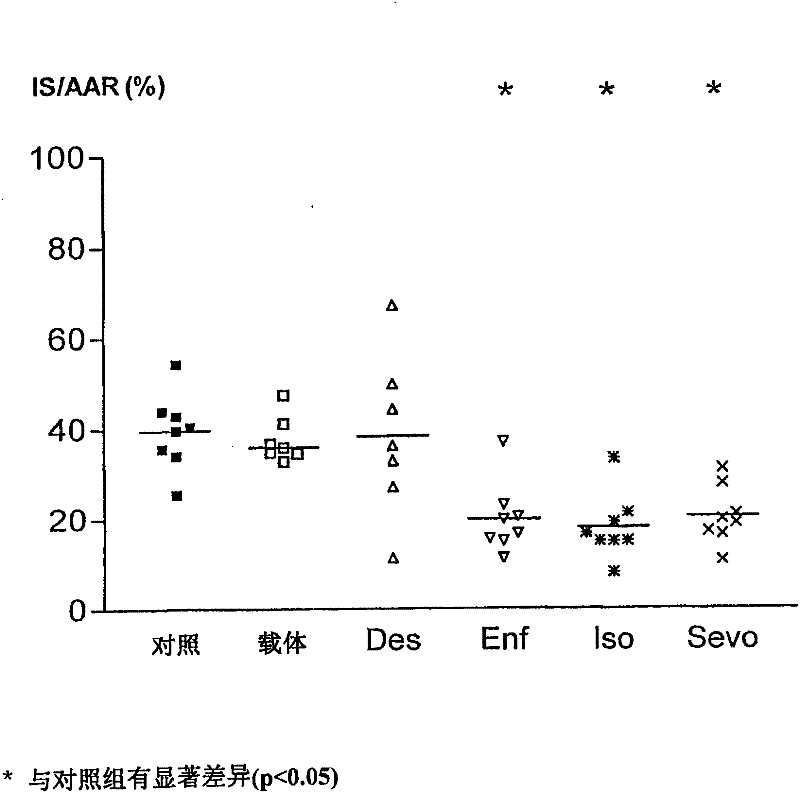
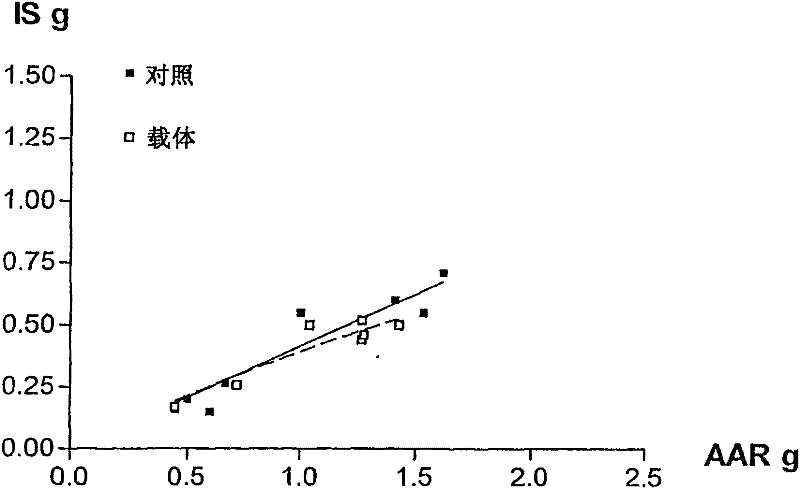
[0096] Embodiment 2: Intravenous use of desflurane, enflurane, isoflurane and sevoflurane on myocardial infarction in rabbits cumulative impact
[0097] These experiments were designed to demonstrate the effect of intravenous administration of different HVAs (enflurane, isoflurane, sevoflurane, and desflurane) in emulsion on myocardial infarct size after ischemia and reperfusion in rabbits. routine preparation
[0098] Male New Zealand White rabbits weighing between 2.5 and 3.0 kg were anesthetized with intravenous sodium pentobarbital (30 mg / kg). Additional doses of pentobarbital were titrated as needed to ensure loss of foot and eyelid reflexes throughout the trial. A tracheotomy is performed through a midline incision in the abdomen, and the trachea is catheterized. Using an air-oxygen mixture (FiO 2 = 0.33) to ventilate the rabbits. Maintain arterial blood gas tension and acid-base status within normal physiological ranges (pH 7.35-7.45, PaCO 2 25-40mmHg, and PaO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com