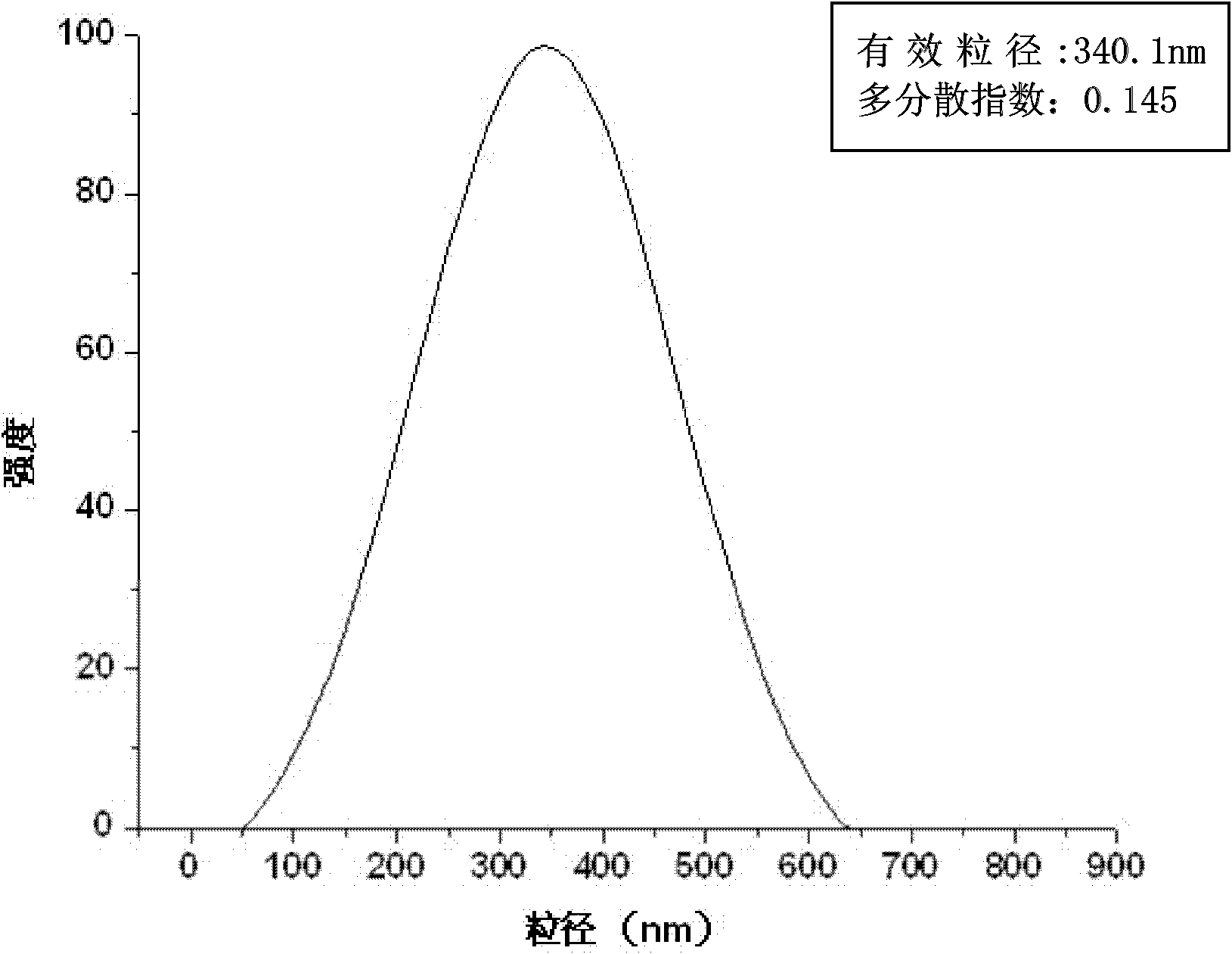
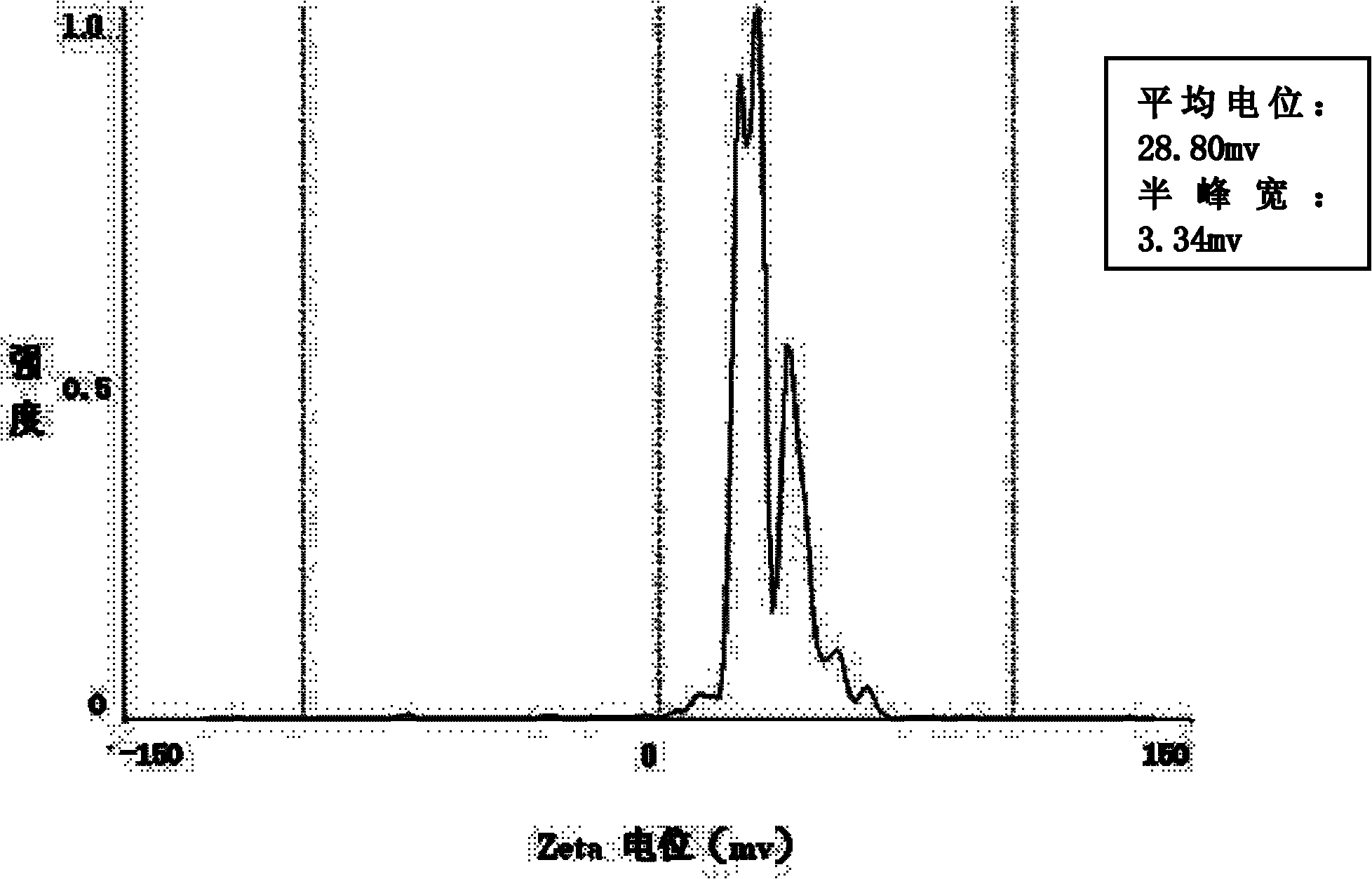
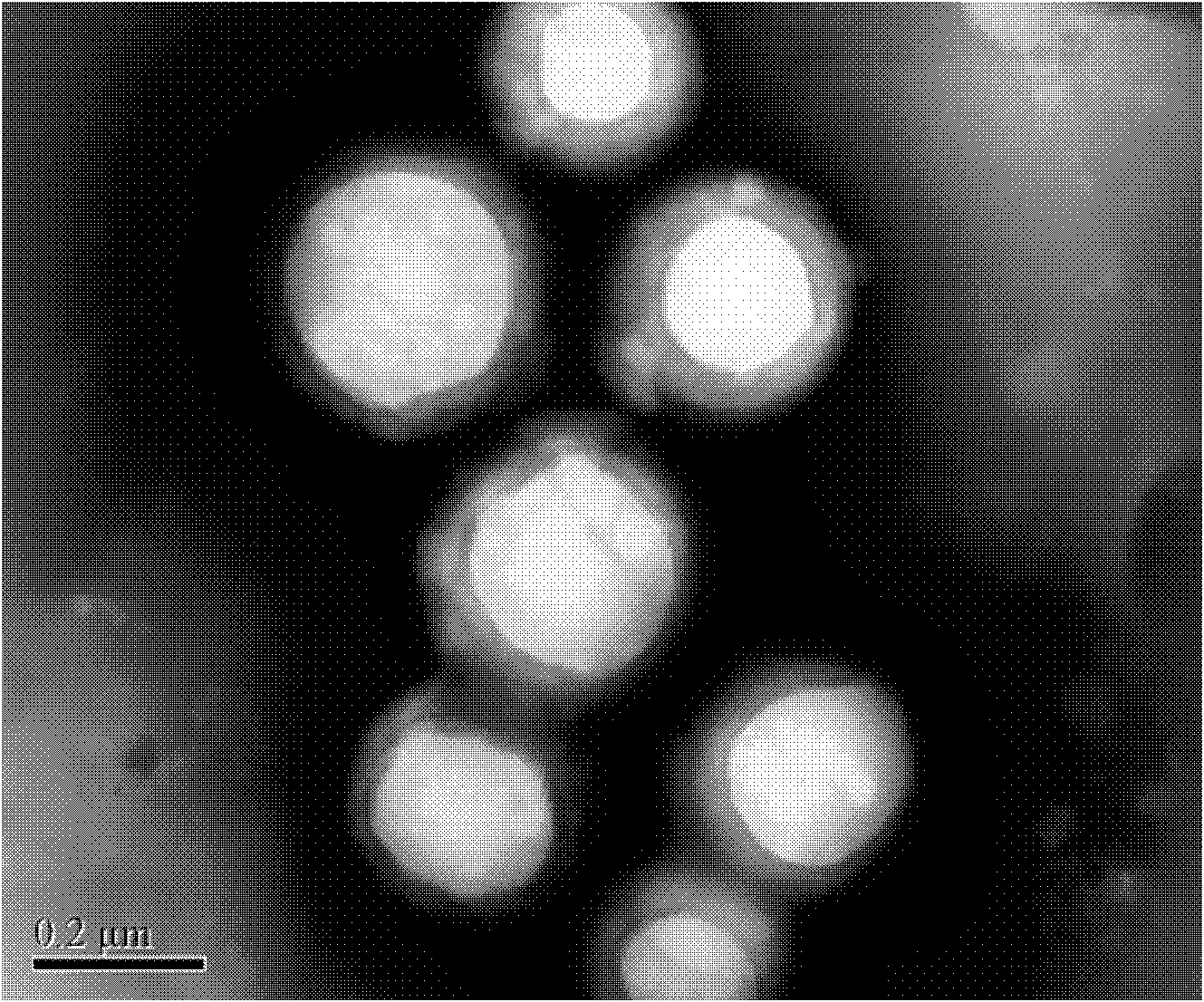Composite drug carried microsphere, minocycline hydrochloride nano controlled-release composite drug carried microsphere system and preparation method thereof
A technology of minocycline hydrochloride and drug-loaded microspheres, which is applied in the field of medicine, can solve the problems of increased cytotoxicity, decreased drug-loading rate, poor hydrophilicity and the like, and achieves a simple and fast preparation process, reduced toxicity and strong antibacterial activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Raw material mass ratio is as follows:
[0055] PLA-b-PGA:PVA=1:2
[0056] PEG-OQCMC:OQCMC=1:2
[0057] Total copies of PEG-OQCMC and OQCMC: Chol=2:1
[0058] Cationic polymer liposomes: PLGA microspheres = 1:1
[0059] (1) Pour 5ml of PVA solution with a concentration of 40mg / ml into a beaker, perform ultrasonication with an ultrasonic cell pulverizer with a power of 200W, add 5ml of a PLA-b-PGA solution with a concentration of 20mg / ml, and continue ultrasonication. Uniform opalescent liquid can be stopped, so as to prepare PLGA microspheres. After magnetic stirring for 24 hours, wash with distilled water at least three times, and vacuum freeze-dry.
[0060] (2) Weigh 10 mg PEG-OQCMC, 20 mg OQCMC, and 15 mg Chol, and dissolve them in 4 ml of dichloromethane. The mixture was placed in an eggplant-shaped bottle, and was rotated at 35° C. on a rotary evaporator at a rotational speed of 50 r / min, and at the same time, a nitrogen stream was passed into the rotary evapo...
Embodiment 2
[0063] Raw material mass ratio is as follows:
[0064] PLA-b-PGA:PVA=1:1
[0065] PEG-OQCMC:OQCMC=1:1
[0066] Total copies of PEG-OQCMC and OQCMC: Chol=4:1
[0067] Cationic polymer liposomes: PLGA microspheres = 2:1
[0068] (1) Pour 5ml of PVA solution with a concentration of 20mg / ml into a beaker, perform ultrasonication with an ultrasonic cell pulverizer at a power of 110W, add 5ml of a PLA-b-PGA solution with a concentration of 20mg / ml, and continue ultrasonication. Uniform opalescent liquid can be stopped, so as to prepare PLGA microspheres. After magnetic stirring for 24 hours, wash with distilled water at least three times, and vacuum freeze-dry.
[0069] (2) Weigh 20mg PEG-OQCMC, 20mgOQCMC, 10mgChol, and dissolve in 4ml dichloromethane. The mixture was placed in an eggplant-shaped bottle, and was rotated at 40° C. on a rotary evaporator at a rotational speed of 45 r / min, and at the same time, nitrogen flow was passed into the rotary evaporator for protection. A...
Embodiment 3
[0072] Raw material mass ratio is as follows:
[0073] PLA-b-PGA:PVA=1:1.5
[0074] PEG-OQCMC:OQCMC=2:1
[0075] Total copies of PEG-OQCMC and OQCMC: Chol=2.5:1
[0076] Cationic polymer liposomes: PLGA microspheres = 1.4:1
[0077] (1) Pour 5ml of PVA solution with a concentration of 30mg / ml into a beaker, perform ultrasonication with an ultrasonic cell pulverizer at a power of 150W, add 5ml of a PLA-b-PGA solution with a concentration of 20mg / ml, and continue ultrasonication. Uniform opalescent liquid can be stopped, so as to prepare PLGA microspheres. After magnetic stirring for 24 hours, wash with distilled water at least three times, and vacuum freeze-dry.
[0078] (2) Weigh 20 mg PEG-OQCMC, 10 mg OQCMC, and 12 mg Chol, and dissolve them in 4 ml of dichloromethane. The mixture was placed in an eggplant-shaped bottle, and was rotated at 32° C. on a rotary evaporator at a rotational speed of 55 r / min, and at the same time, nitrogen flow was passed into the rotary evapo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com