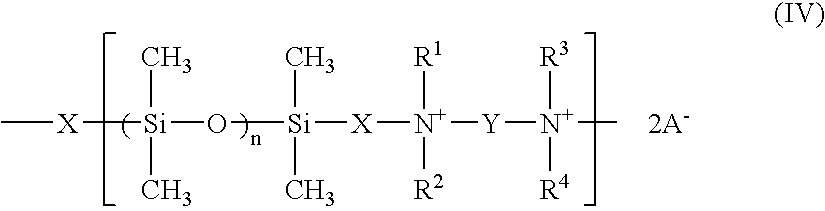
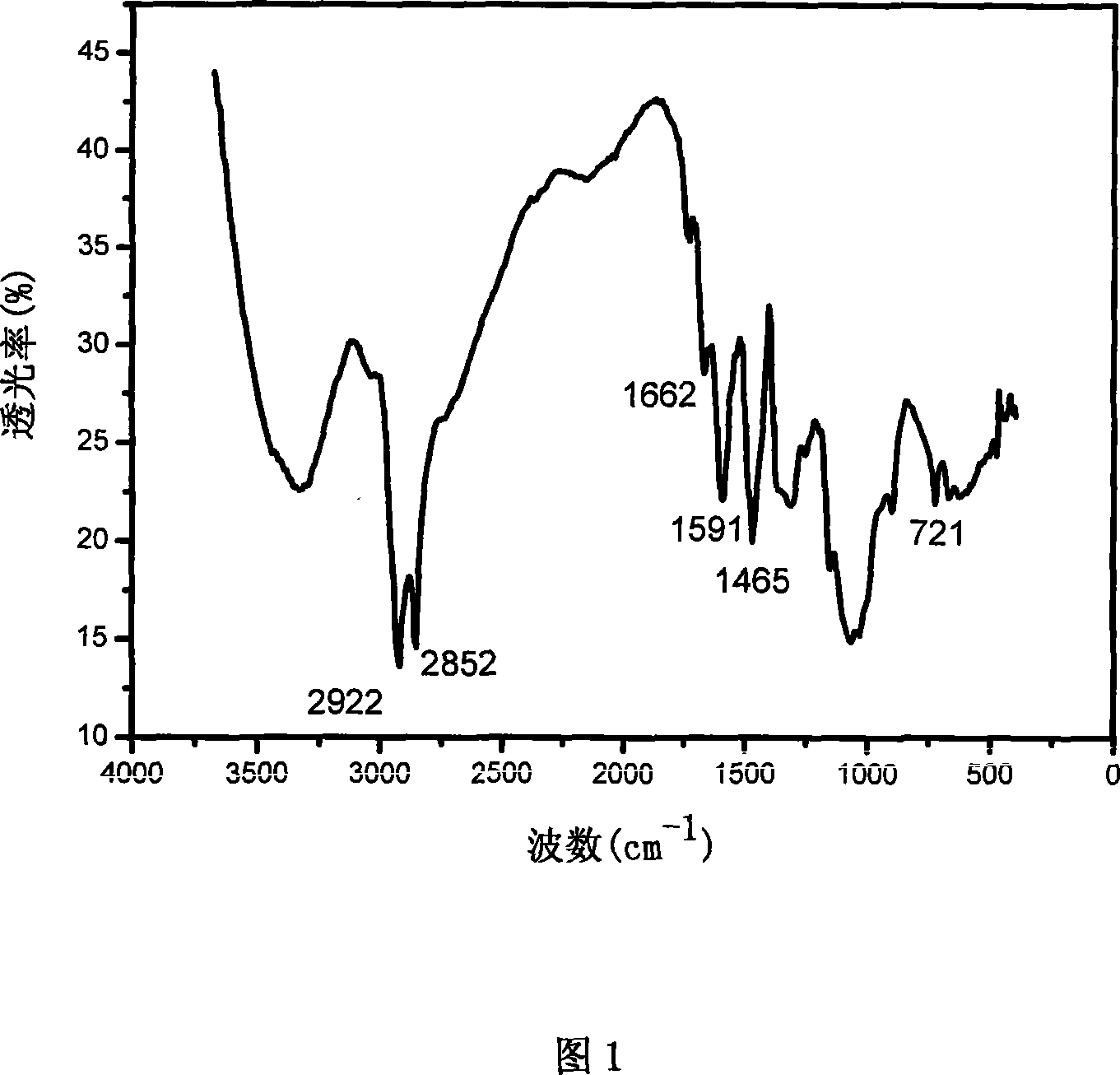
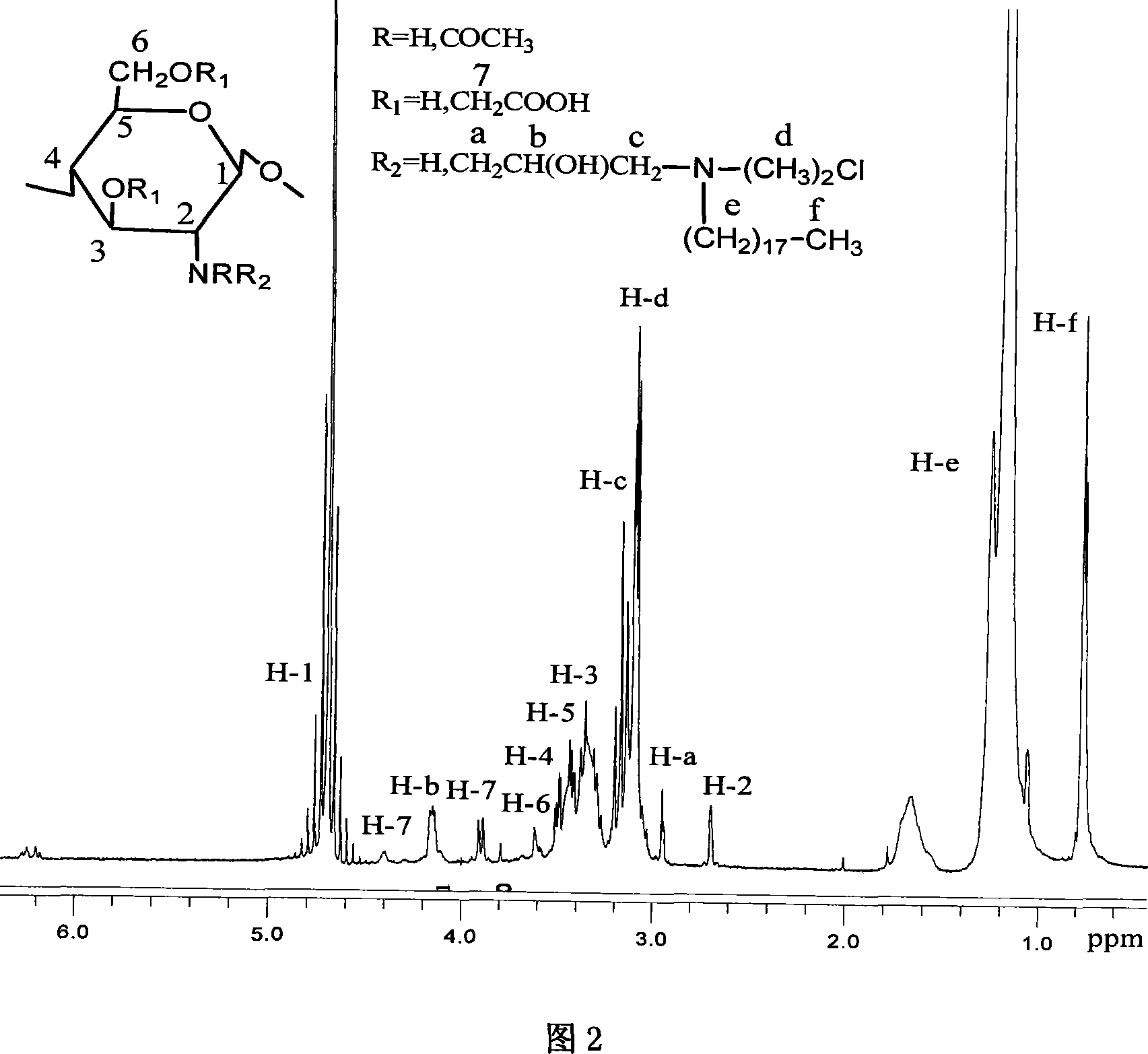
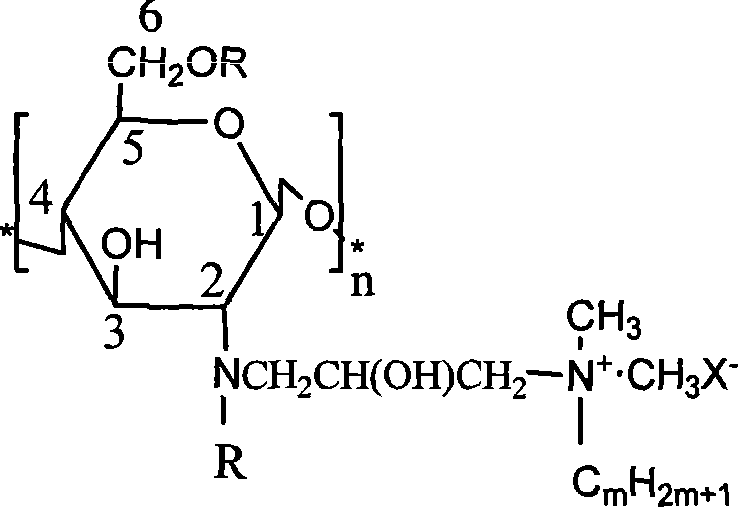
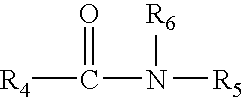
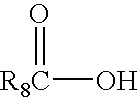
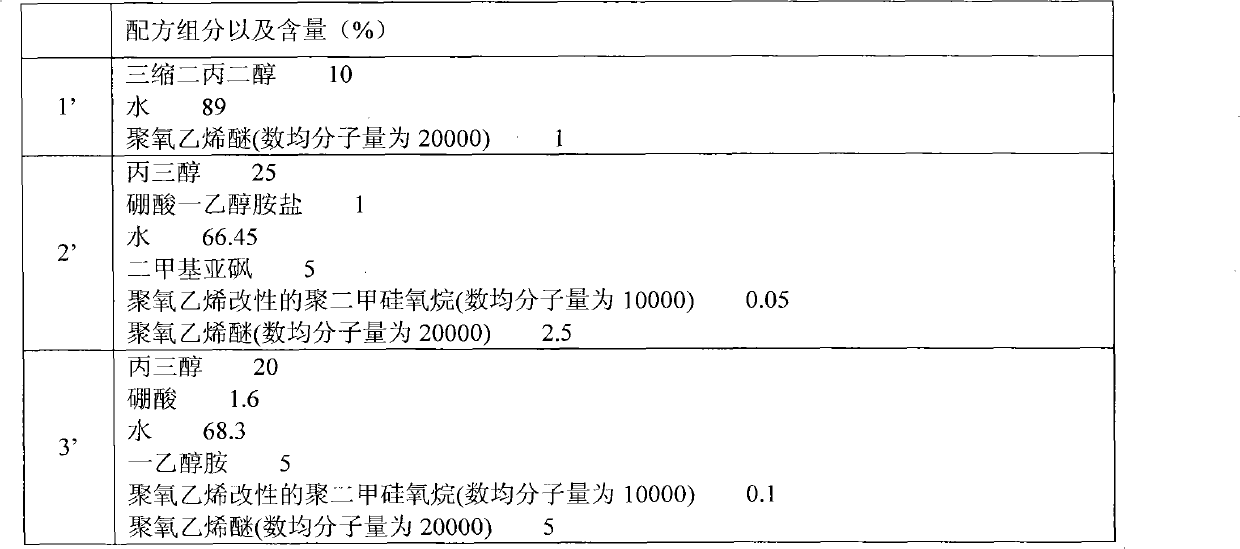
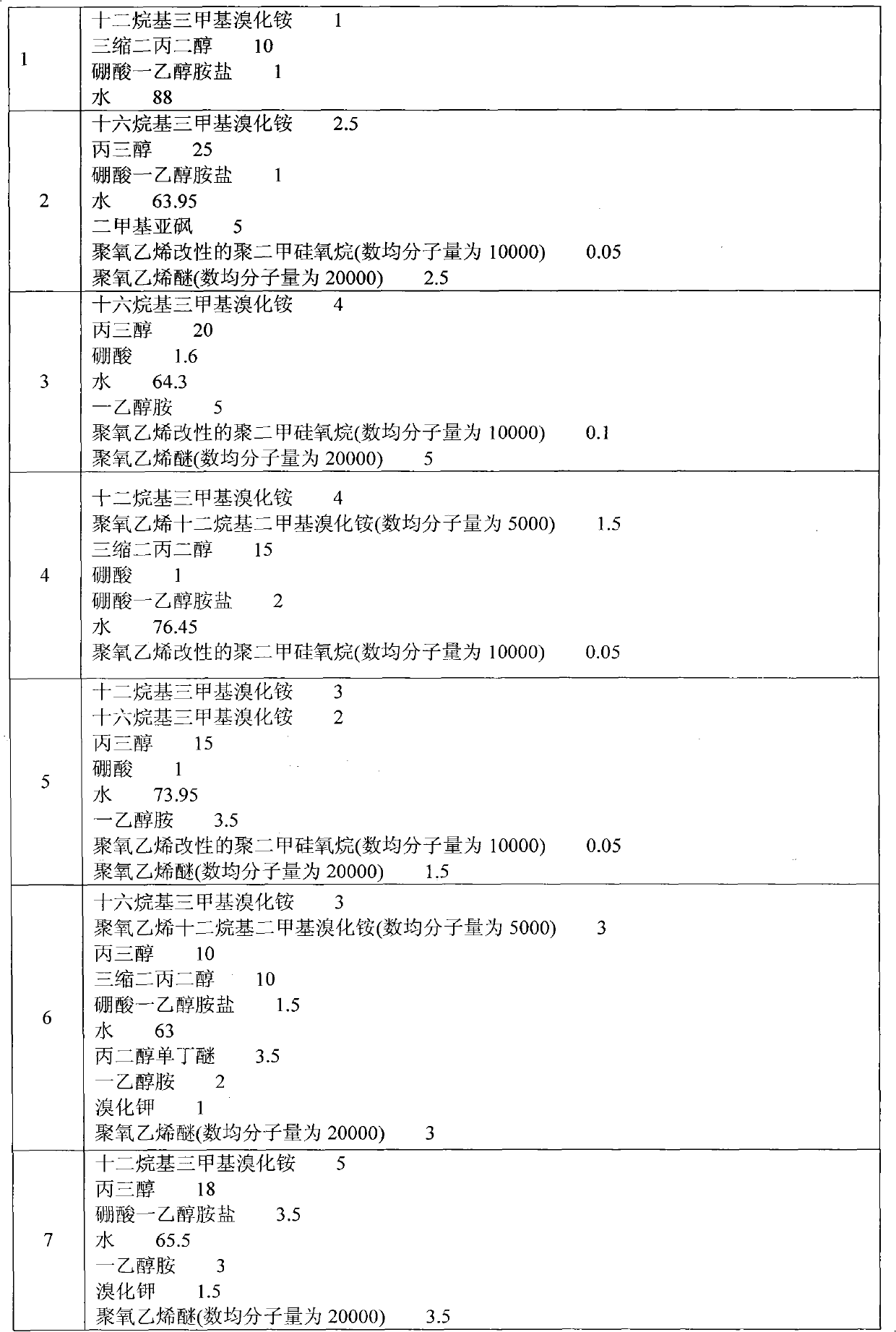
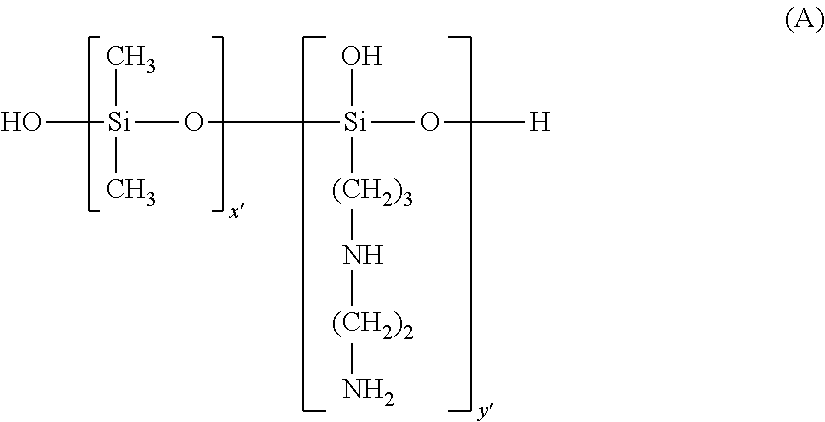
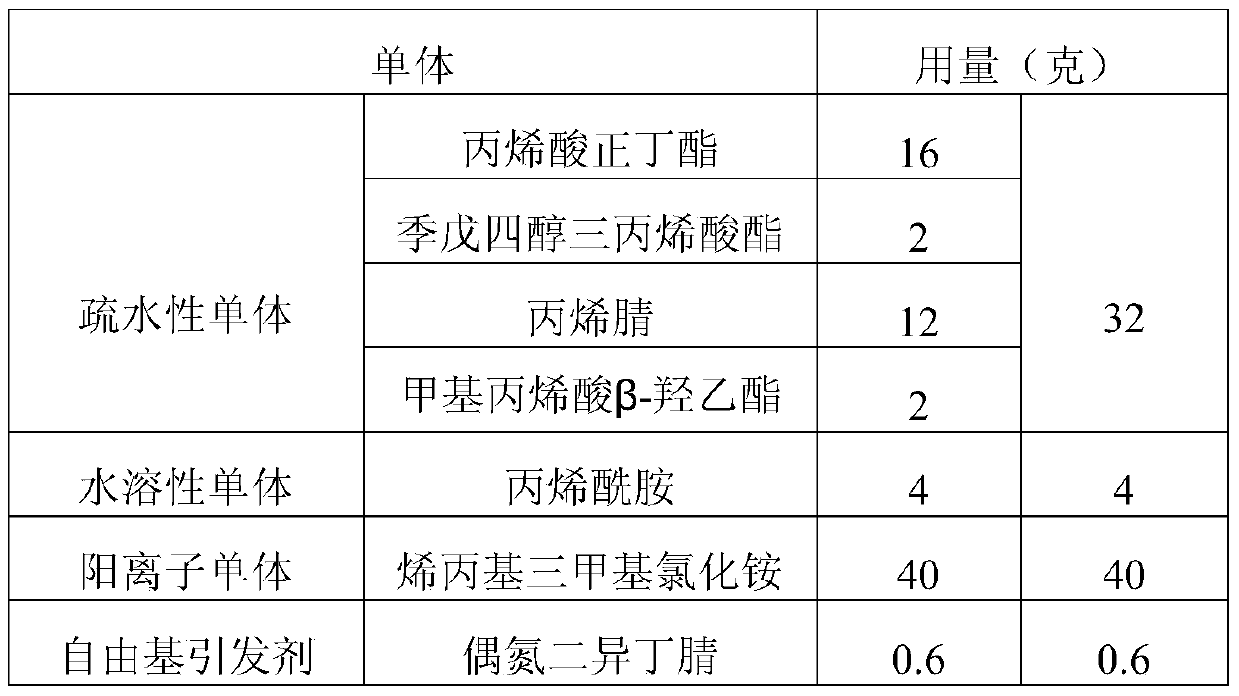
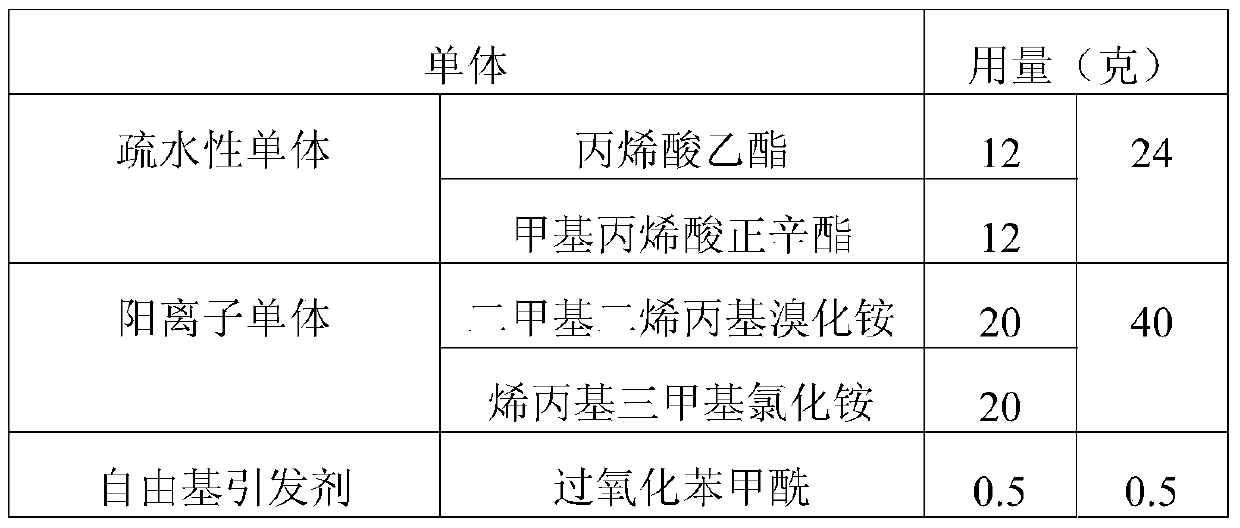
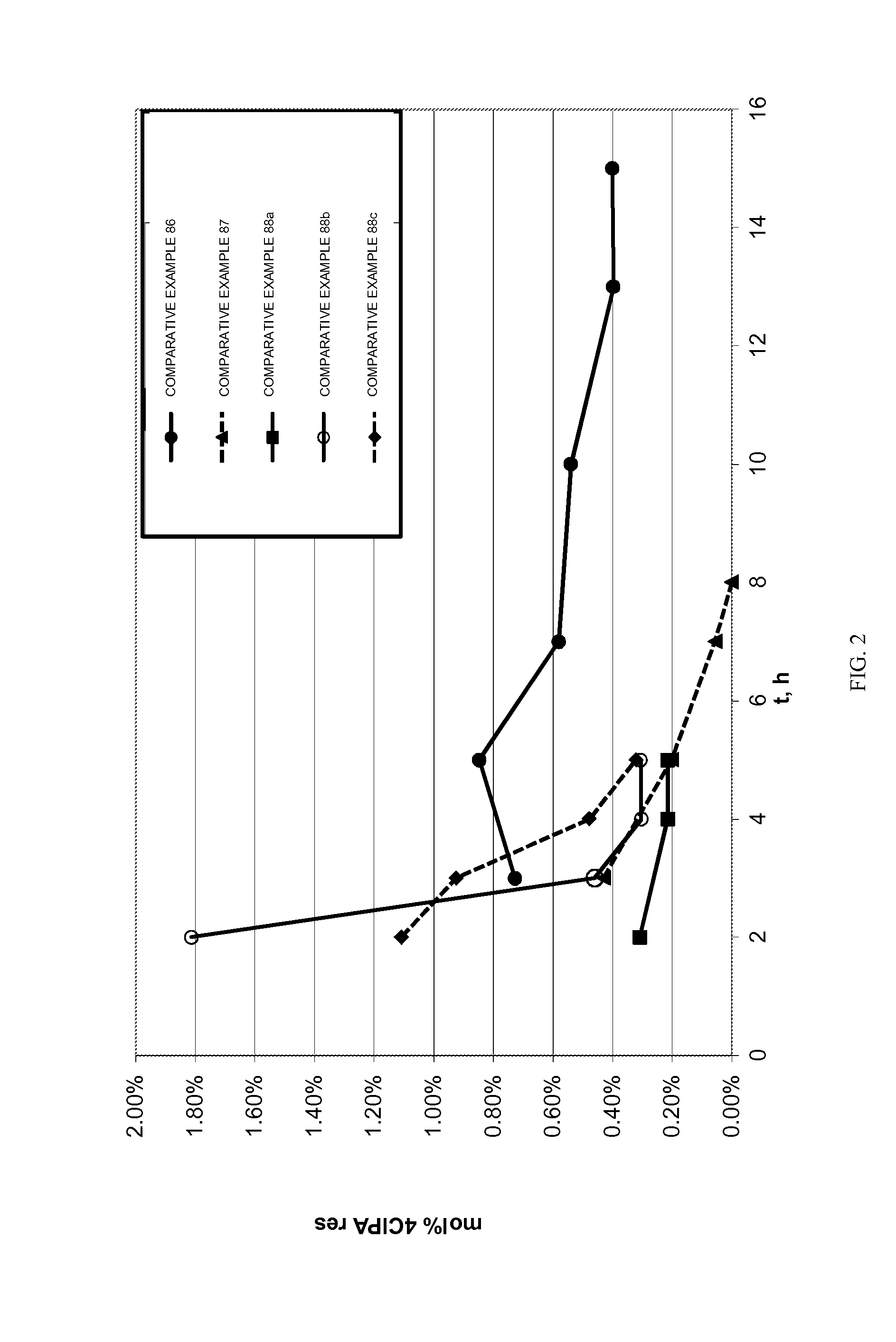
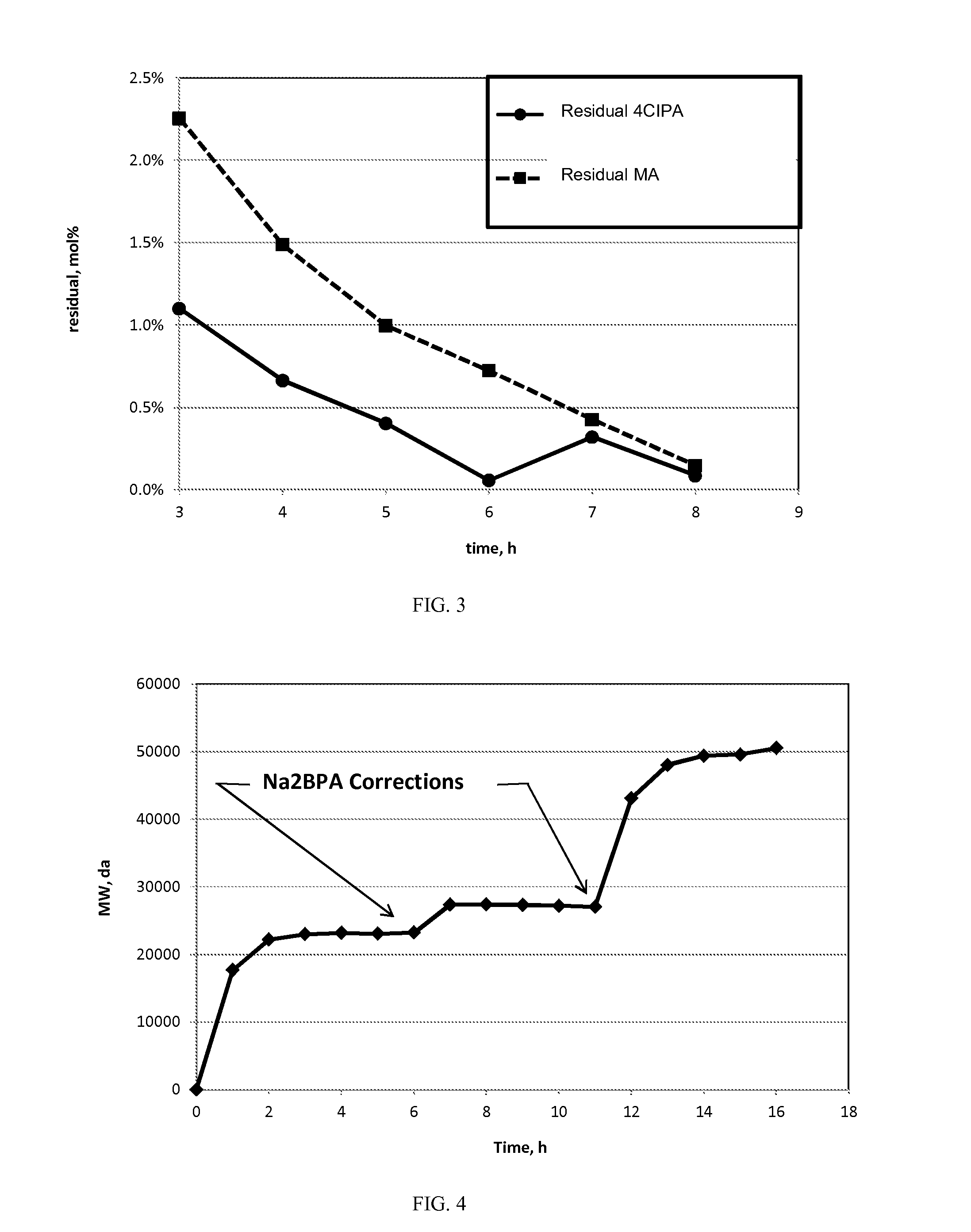
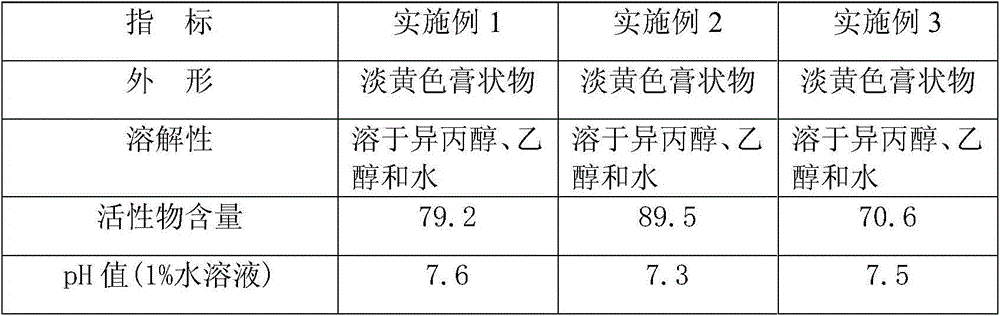
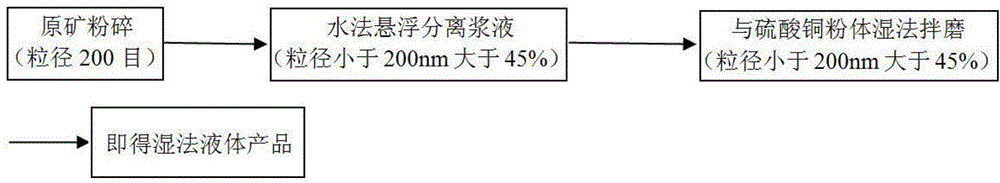
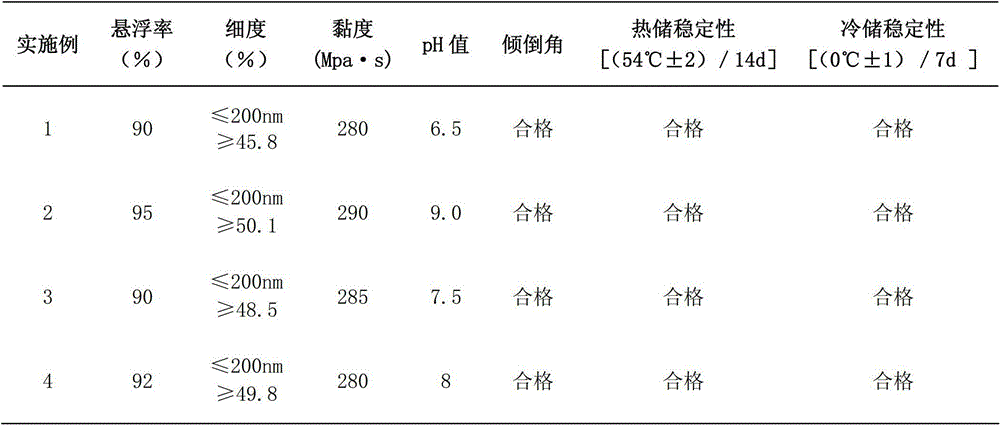
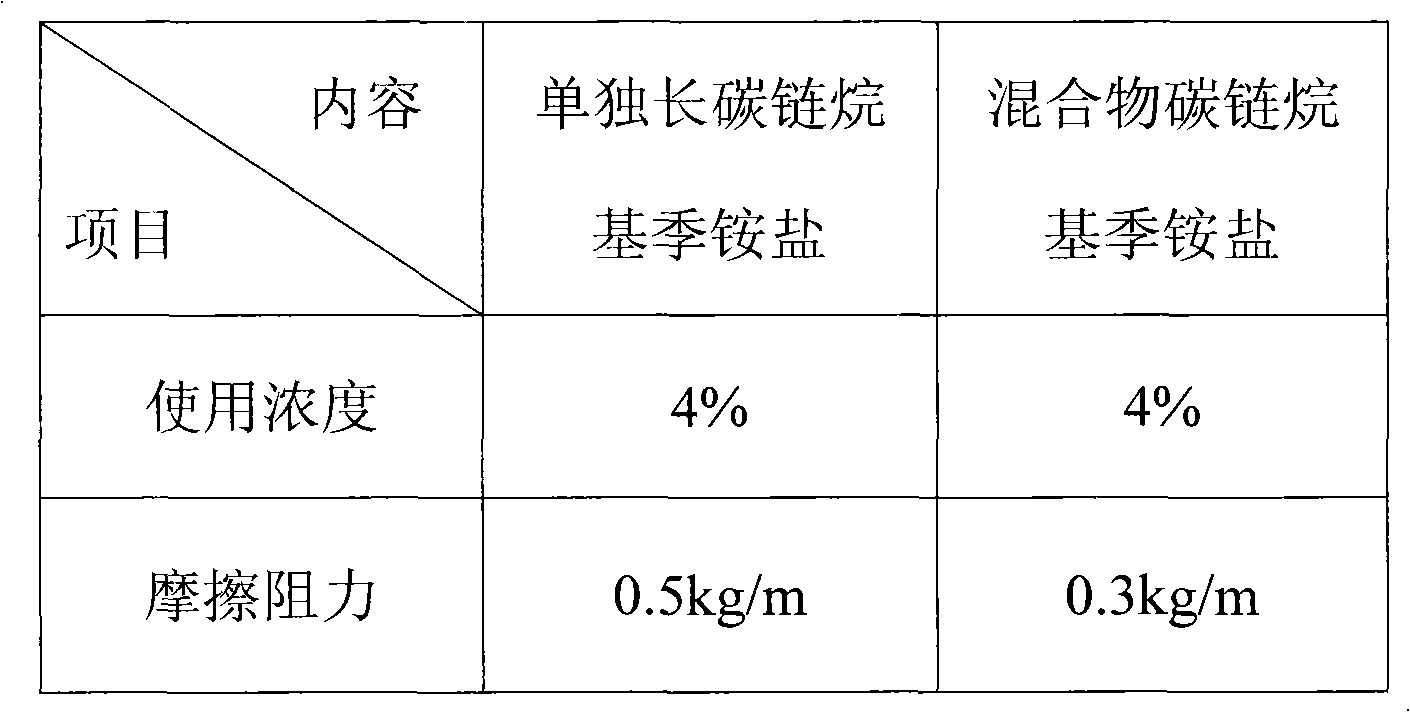
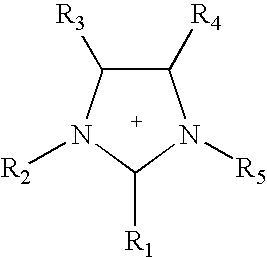
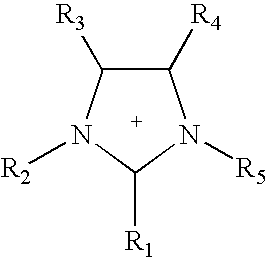
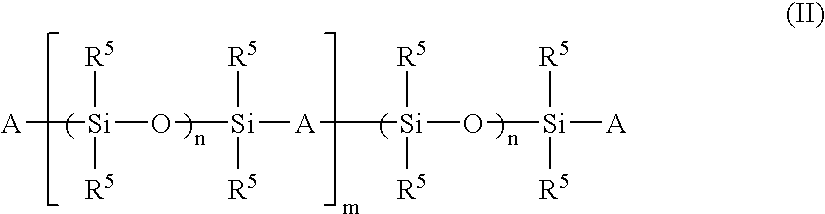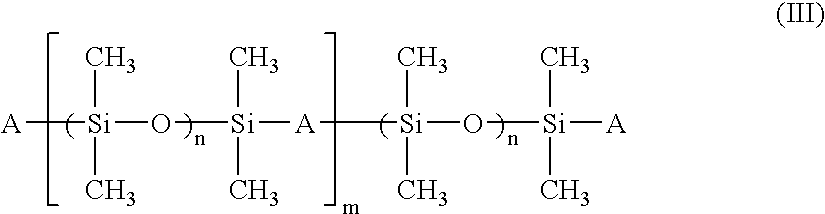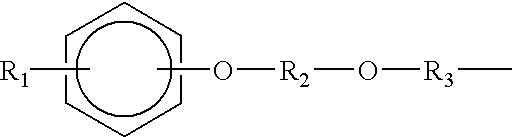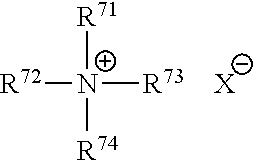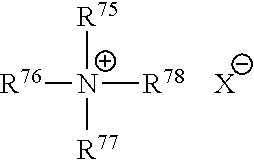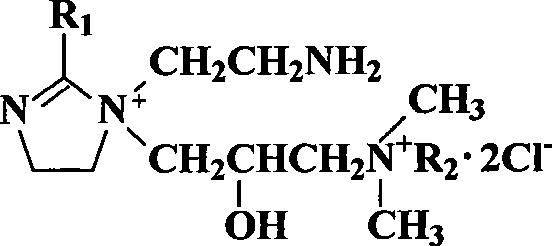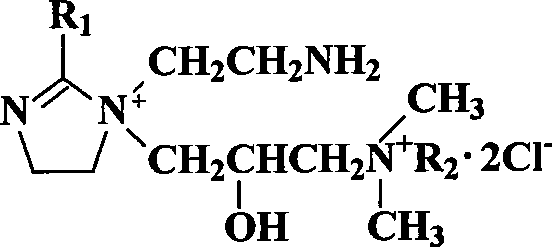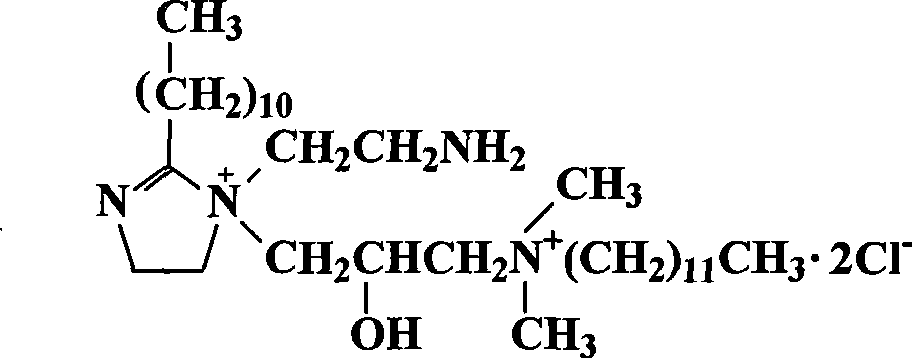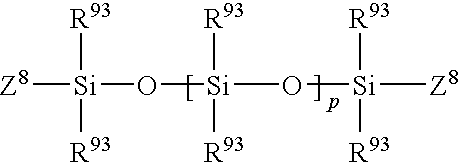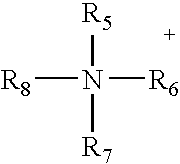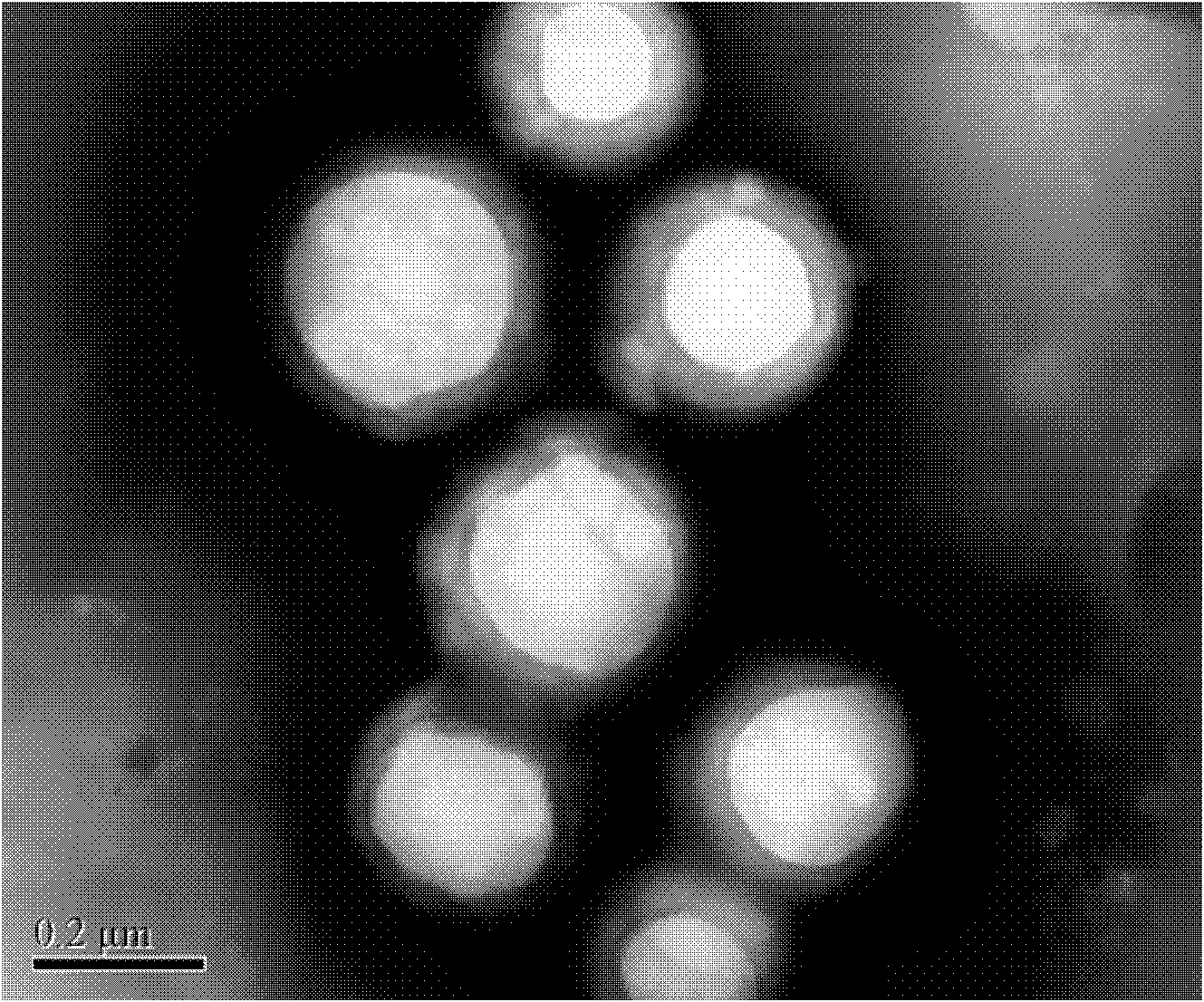Patents
Literature
279 results about "Alkyl quaternary ammonium chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hair conditioning composition comprising quaternized silicone polymer, grafted silicone copolyol, and dialkyl cationic surfactant
ActiveUS20080292575A1Improved conditioning benefitReduced friction in conditioningCosmetic preparationsHair cosmeticsPolyolSURFACTANT BLEND
Disclosed is a hair conditioning composition comprising: (a) a quaternized silicone polymer; (b) a grafted silicone copolyol; (c) a cationic surfactant system comprising a dialkyl quaternized ammonium salt cationic surfactant; (d) a high melting point fatty compound; and (e) an aqueous carrier. The present invention can provide improved conditioning benefits such as smooth feel and reduced friction in conditioning compositions using dialkyl quaternized ammonium salt cationic surfactants.
Owner:THE PROCTER & GAMBLE COMPANY
Amphiphilic chitosan quatermary ammonium salt with long alkane radical and its prepn
The present invention relates to one kind of amphiphilic chitosan quaternary ammonium salt with long alkane radical and its preparation process. The amphiphilic chitosan quaternary ammonium salt with long alkane radical is prepared with water soluble chitosan derivative as modified target and through epoxy long chain alkyl quaternary ammonium salination, and has high solubility. Compared with available technology, the present invention has the features of simple preparation process suitable for industrial production, capacity of using the product as gene medicine carrier, emulsifier and antiseptic, and wide application of the product in medicine, daily chemical, etc.
Owner:TIANJIN UNIV
Transdermal compositions
The present invention is directed to transdermal compositions and the uses thereof. These compositions include at least one of the following components: a C1-C6 dialkyl, C12-C30 dialkyl quaternary ammonium salt, a C12-C30 fatty acid, a nitrogenous organic base, C12-30 fatty alcohol, monoglyceride or the reaction products thereof.
Owner:MICRODERMIS CORP
Composite bactericide containing dialkyl quaternary ammonium salt and its application
InactiveCN1426681AReduce use costExcellent peelabilityBiocideAnimal repellantsIsothiazolinoneDisinfectant
A composite disinfectant containing dialkyl quaternary ammonium salt for disinfecting the circulated cooling water contains at least one dialkyl quaternary ammonium salt disinfectant and at least one non-oxide disinfectant chosen from frequency used quaternary ammonium salt, isothiazolinone, glutaraldehyde, and bithiocyanomethane.
Owner:RES INST OF BEIJING YANSHAN PETROCHEM
Water-based glass grinding fluid
InactiveCN102559354AImprove cooling effectImprove the lubrication effectLubricant compositionAlcoholActive agent
The invention discloses a water-based glass grinding fluid which comprises a brominated alkyl quaternary ammonium salt surfactant, organic alcohol, a corrosion inhibitor and water. The water-based glass grinding fluid has excellent properties of cooling, lubricating, washing, corrosion preventing and the like, and has a better chemical dressing function on a diamond grinding tool; and according to the water-based glass grinding fluid, the grinding precision can be remarkably improved, the service life of the grinding tool is prolonged, and the greasy blockage of the grinding tool is effectively prevented. The water-based glass grinding fluid has good glass grinding efficiency, excellent chemical stability and low cost, has no harm to the health, does not pollute the environment, is convenient for use, and can be widely applied to the grinding and cleaning processes of various kinds of glass.
Owner:ANJI MICROELECTRONICS (SHANGHAI) CO LTD
High-dispersity acidic copper plating additive as well as preparation method and application thereof
InactiveCN105734623AImprove ductilityImprove current efficiencyPrinted element electric connection formationDispersityCopper plating
The invention relates to the field of highly dispersive all-bright acidic copper plating of printed circuit boards, in particular to a highly dispersive acidic copper plating additive and its preparation method and application. A highly dispersed acidic copper plating additive: at least one divalent sulfide main brightener, at least one polyethylene glycol or polypropylene glycol or both block copolymer polymer inhibitors, at least one alkyl quaternary ammonium salt Type cationic surfactant, 5‑50ml / L sulfuric acid, 0.1‑4ml / L formaldehyde. Its preparation method is to slowly add a specified amount of concentrated sulfuric acid into water, stir evenly and cool to room temperature, then sequentially add the inhibitor, surfactant, main brightener and formaldehyde into the sulfuric acid aqueous solution, stir until completely dissolved Concentrate to the final volume to obtain the highly dispersed acidic copper plating additive of the present invention. The high-dispersion acidic copper plating additive of the invention is especially suitable for electroplating through-holes with high thickness-to-diameter ratio of printed circuit boards, and can obviously improve the dispersion ability of the plating solution.
Owner:GUANGDONG LEAR ELECTROCHEM LTD
Amphiprotic-Gemini type cationic composite adsorbent, and preparation method thereof
ActiveCN103752274AIncrease organic carbon contentImprove adsorption capacityOther chemical processesWater contaminantsClay mineralsBetaine
The invention discloses an amphiprotic-Gemini type cationic composite adsorbent, and a preparation method thereof. The amphiprotic-Gemini type cationic composite adsorbent is an expansive clay mineral modified by an amphiprotic surfactant and a Gemini type cationic surfactant; the amphiprotic surfactant is alkyl dimethyl betaine, and alkyl carbon chain of alkyl dimethyl betaine possesses 10 to 18 carbon atoms; the Gemini type cationic surfactant is an alkyl quaternary ammonium gemini surfactant, and alkyl carbon chain of the alkyl quaternary ammonium gemini surfactant possesses 10 to 18 carbon atoms. Adsorption capacities of the amphiprotic-Gemini type cationic composite adsorbent obtained via modification on organic pollutants and heavy metal anions are improved, so that a problem that absorption capacity of amphiprotic organic modified soil on pollutants and heavy metal anions is poor is solved. The preparation method of the amphiprotic-Gemini type cationic composite adsorbent is simple, and operation is convenient.
Owner:NORTHWEST A & F UNIV
Compositions comprising an associaton of cationic compounds, silane compounds, esters and fatty substances
Disclosed are compositions for treating and conditioning keratinous substrates, comprising a first cationic surfactant chosen from alkyl quaternary ammonium or diammonium salts; a second cationic surfactant chosen from alkyl amines or alkyl amine salts; a first silane compound, a cationic vinylpyrrolidone polymer, fatty alcohols, glyceryl esters, organic acids, and water. Also disclosed are methods treating and conditioning keratinous substrates using the composition.
Owner:LOREAL SA
Modified bentonite load nanometer iron material and preparation method thereof
InactiveCN103464091AImprove adsorption capacityStrong positiveOther chemical processesWater/sewage treatment by sorptionIron saltsFiltration
The invention relates to a modified bentonite load nanometer iron material and a preparation method thereof. According to the technical scheme, the preparation method includes the steps of stirring bentonite and bivalent iron salt solutions to prepare the mixed liquor of the bentonite and the bivalent iron salt solutions, wherein the mass ratio between an iron element and the bentonite is 1: (1-2), then dropwise adding reducing agent solutions to the mixed liquor of the bentonite and the bivalent iron salt solutions and stirring to prepare mixed liquor containing bentonite load nanometer iron, and adding alkyl quaternary ammonium salt solutions into the mixed liquor containing the bentonite load nanometer iron and stirring, wherein the volume ratio between the mixed liquor containing the bentonite load nanometer iron and modifying agent solutions is 1: (0.15-0.20), and the steps are all carried out in a nitrogen environment; then carrying out suction filtration, drying and grinding the obtained solid in a vacuum environment to obtain the modified bentonite load nanometer iron material. The modified bentonite load nanometer iron material prepared by the preparation method has strong reducing capacity and adsorption capacity, and is suitable for removing heavy metal chromium (VI) and heavy metal arsenic (V), wherein the heavy metal chromium (VI) and the heavy metal arsenic (V) exist in water in an anionic form.
Owner:WUHAN UNIV OF SCI & TECH
Shampoo composition provided with anti-dandruff and opsonize efficacy
The present invention provides a shampoo compound which has the effects of scurf removal and conditioning. The shampoo compound of the present invention includes the following components with the weight percentages: a) 5 percent to 50 percent surfactant; b) 0.01 percent to 10 percent alkyl propyl dimethyl 2, 3 dihydroxy ammonium chloride alkyl quaternary ammonium salts; c) 0.01 percent to 10 percent non-volatile siloxane; d) 0.01 percent to 3 percent cationic polymer; e) 0.01 percent to 5 percent scurf-removing agent and f) deionized water. Compared with other scurf-removing shampoos, the shampoo compound of the present invention has better effect conditioning of the scalp and hair and better scurf-removing and itch reliving effects.
Owner:BEIERSDORF AG
Conditioning composition comprising dual cationic surfactant system, aminosilicone and silicone resin
InactiveUS20100015078A1Reliable conditionsCosmetic preparationsHair cosmeticsAminosilochromeSilorane Resins
Disclosed is a conditioning composition comprising: (a) from about 0.1% to about 10% of a surfactant system comprising: di- and mono-alkyl quaternized ammonium salt cationic surfactants; (b) from about 1% to about 15% of a high melting point fatty compound; (c) from about 0.1 % to about 20% of an aminosilicone; (d) from about 0.0001% to about 10% of a silicone resin; and (e) an aqueous carrier. The composition of the present invention can provide improved wet and dry conditioning benefits while providing chronic / long lasting color protection benefits.
Owner:THE PROCTER & GAMBLE COMPANY
Cationic flocculant and preparation method thereof
InactiveCN105504163AGood electrostatic attractionHigh affinityWater contaminantsWater/sewage treatment by flocculation/precipitationCyclohexanonePersulfate
The invention discloses a cationic flocculant and a preparation method thereof. The cationic flocculant is prepared by carrying out polymerization reaction on reaction monomers comprising a cationic monomer and a hydrophobic monomer under the action of a free-radical initiator, wherein the cationic monomer comprises one or combination of more of acryloyloxyethyl alkyl quaternary ammonium salts and / or allyl alkyl quaternary ammonium salts; the hydrophobic monomer comprises one or combination of more of styrene, acrylonitrile, vinyl acetate, vinyltrimethoxysilane, vinyltriethoxysilane and acrylate; and the free-radical initiator comprises one or combination of more of azodiisobutyronitrile, azodiisohexyl cyanide, azodiisobutylamidine hydrochloride, cyclohexanone peroxide, benzoperoxide and persulfate. The flocculant can adsorb anionic dyes, has favorable affinity with electrically neutral organic matters or organic matters with a small amount of anions, and has excellent flocculating settling effect.
Owner:GUANGDONG ESQUEL TEXTILES CO LTD
Quaternary cationic polymers
ActiveUS20180163020A1Avoid corrosionBiocideScale removal and water softeningIsobutanolEthyleneglycol monobutyl ether
A cationic polymer salt composition is provided that includes a reaction product derived from reaction of a polyamine or a polyalkyleneimine and a substituted alkyl trialkyl quaternary ammonium salt. Also provided are surfactant compositions. The compositions may also include carriers, such as water, methanol, ethanol, propanol, isopropanol, butanol, isobutanol, monoethyleneglycol, an ethyleneglycol monobutyl ether, and hexylene glycol.
Owner:ECOLAB USA INC
Method for preparing monodisperse mesoporous bioactive glass microspheres through template method
ActiveCN103342453AImprove performanceGreat application potentialGlass shaping apparatusPharmaceutical non-active ingredientsAlkaline earth metalMicrosphere
The invention relates to a method for preparing monodisperse mesoporous bioactive glass microspheres through a template method, belonging to the field of biomedical materials. A silicon source, a phosphorus source, a calcium source, an iron source, an alkali metal or alkaline-earth metal source is adopted as a raw material, a triblock copolymer or alkyl quaternary ammonium salt type cation is taken as a surfactant, and an in-situ sol-gel method is performed in a three-dimensional ordered macroporous polymer or a carbon template to synthesize the ordered mesoporous bioactive glass microspheres. The bioactive glass microspheres prepared according to the invention have the characteristics of good biocompatibility, biodegradability, ordered mesoporous structure, higher specific surface area, magnetism, monodispersibility of particles, environmental stability and the like; and in comparison with various medicament delivery carrier materials which are extensively researched currently, the bioactive glass microspheres have more comprehensive advantages and further have broad application prospects as novel medicament controlled release carrier materials.
Owner:扬州智创企业运营管理服务有限公司
Polyetherimide compositions, methods of manufacture, and articles formed therefrom
A method for the manufacture of a polyetherimide composition includes catalyzing the reaction of a dianhydride and an organic diamine with a catalyst selected from guanadinium salts, pyridinium salts, imidazolium salts, tetra(C6-24)aryl ammonium salts, tetra(C7-24 arylalkylene)ammonium salts, dialkyl heterocycloaliphatic ammonium salts, bis-alkyl quaternary ammonium salts, (C7-24arylalkylene)(C1-16alkyl)phosphonium salts, (C6-24aryl)(C1-16alkyl)phosphonium salts, phosphazenium salts and combinations thereof, optionally in the presence of a solvent.
Owner:SABIC GLOBAL TECH BV
Multi-functional antistatic agent
ActiveCN104018345AImprove antistatic performanceWide range of usesFibre treatmentCoatingsCarbon numberFiber
The invention discloses a multi-functional antistatic agent. The multi-functional antistatic agent is prepared from the following components in percentage by weight: 40-60% of fatty amine polyoxyethylene ether, 20-40% of alkyl quaternary ammonium salt, and 10-30% of alcohols solvent, wherein the fatty amine of the fatty amine polyoxyethylene ether is processed by natural fat and has the carbon chain length of C16-18, the fatty amine can be cocoanut oil amine and tallow amine, the alkyl quaternary ammonium salt has alkyl carbon chain length of C2-18, and can be dioctadecyl alkyl quaternary ammonium salt and octadecyl alkyl quaternary ammonium salt, and the carbon number of the alcohols solvent is 2-4, and the alcohols solvent can be ethyl alcohol, isopropyl alcohol or butyl alcohol. The multi-functional antistatic agent is compounded by cationic and non-ionic antistatic agents, has the advantages of the cationic antistatic agent such as good antistatic performance and wide application, also has the advantages of the non-ionic antistatic agent such as good stability and long efficiency, can be diluted by solvent for fiber and plastic surface spraying, also can be used for fiber oil formula, and can be added into resin for achieving long-acting antistatic effect.
Owner:湖北尚助化学有限公司
Imidazoline asymmetrical bi-quaternary ammonium salt, method for preparing same and application thereof
InactiveCN101531635AInexpensive and readily available biological activityBroad biological activityOrganic chemistrySolubilityDrug biological activity
The invention relates to an imidazoline asymmetrical bi-quaternary ammonium salt. The preparation method comprises the following steps that: diethylene triamine and organic acids of octalkyl to heptadecyl react to generate alkyl imidazoline; long chain alkyl tertiary amine, hydrochloride of long chain alkyl tertiary amine and epichlorohydrin react to generate N-(3-chlorine-hydroxypropyl)-N, N-dimethyl long chain alkyl quaternary ammonium salt; and the alkyl imidazoline and the N-(3-chlorine-hydroxypropyl)-N, N-dimethyl long chain alkyl quaternary ammonium salt react. When the salt is used as a carbon steel corrosion protection acid pickling inhibitor, the salt has the advantages of easy synthesis, low-priced and easily-bought materials; because the salt is the organic inhibitor, the salt is nontoxic and unharmful, and has wide biological activity and good water solubility and can effectively inhibit corrosion of metallic matrix in the acid and excessive consumption of acid liquor; moreover, the salt also has the advantages of low consumption, high efficiency, strong sustained action capability and good application prospect.
Owner:OCEAN UNIV OF CHINA
Illite/montmorillonite mixed-layer clay sterilization suspending agent
InactiveCN102742573APermanent suspensionPermanent dispersionBiocideDisinfectantsPesticide residueSuspending Agents
The invention discloses an illite / montmorillonite mixed-layer clay sterilization suspending agent, which is prepared from the following raw materials in percentage by weight: 40-70 percent of illite clay mineral, 15-40 percent of montmorillonite clay mineral and 5-30 percent of inorganic sterilization additive. Since the raw materials are natural nanomaterials which have the performance of permanent suspension property and dispersibility, and copper sulfate, carboxymethyl cellulose and alkyl quaternary ammonium salt powder are added, the unique dispersibility and strong suspension property of the clay micro / nano level powder in water are kept, and the sterilization capacity is also achieved. The inorganic suspending agent has the characteristics of high efficiency, low toxicity, environmental friendliness and safety, the suspension rate reaches over 90 percent, the dispersibility and the suspension property of the pesticide can be kept for long time, the problem of super-standard pesticide caused by the conventional organic suspending agent is solved, and pollution to environment and serious injury to human bodies caused by pesticide residue in crop products can be effectively reduced.
Owner:上思县文德矿业科技咨询服务有限公司 +2
Hair Conditioning Composition Comprising Cationic Surfactant System and Direct Dye
ActiveUS20100150858A1Good coloring effectImprove efficiencyCosmetic preparationsCationic surface-active compoundsSulfateAlkyl amine
Disclosed is a hair conditioning composition comprising: a cationic surfactant system of either (i) or (ii): (i) the system comprising: a salt of a mono-long alkyl quaternized ammonium and an anion wherein the anion is selected from the group consisting of C1-C4 alkyl sulfate, and mixtures thereof; and a di-long alkyl quaternized ammonium salt; or (ii) the system comprising: salts of mono-long alkyl amines wherein the mono-long alkyl group has 20 to about 24 carbon atoms; and a di-long alkyl quaternized ammonium salt; a high melting point fatty compound; a direct dye; and an aqueous carrier. The composition of the present invention provides improved coloring benefits, while providing conditioning benefits
Owner:THE PROCTER & GAMBLE COMPANY
Harmless clean fracturing fluid
The invention provides a harmless clean fracturing fluid which contains alkyl quaternary ammonium salt mixture, counter-ion salt, low carbon alcohol and water, wherein, the concentration of an aqueous solution is between 2 percent and 6 percent, and the alkyl quaternary ammonium salt mixture: the counter-ion salt: the low carbon alcohol is equal to 6:3:1 calculating by weight. The raw materials of used components are added into water in sequence according to proportion under agitating condition when using the clean fracturing fluid on site, which has higher viscous elasticity and carrying function when being prepared into an aqueous solution of 2 to 6 percent, thus fully meeting the requirements of the site fracturing fluid sand-carrying of an oil-gas field. The invention has the advantages of simple formula, low cost, small fluid loss, high recovery rate of core permeability, residual free and being easy to be emitted, etc.
Owner:陕西延长石油油田化学科技有限责任公司
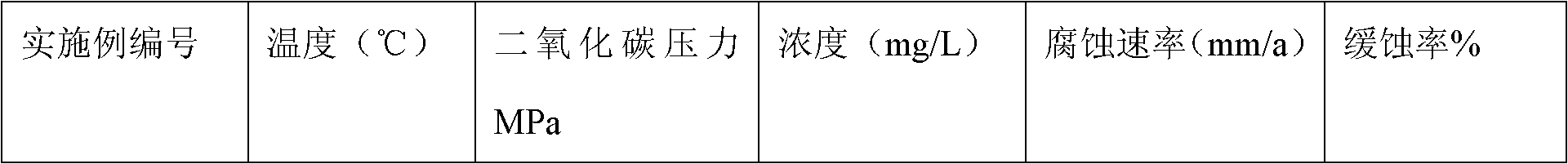
Corrosion inhibitor for inhibiting corrosion of high-temperature high-pressure carbon dioxide and preparation method thereof
The invention provides a corrosion inhibitor for inhibiting the corrosion of high-temperature high-pressure carbon dioxide and a preparation method thereof. The corrosion inhibitor comprises 1 to 80 weight percent of thioimidazolone derivative, 1 to 50 weight percent of quaternary alkylammonium salt, 0.01 to 20 weight percent of sulfur-containing low molecular weight organic matter, 0 to 70 weight percent of water, 0 to 30 weight percent of nonionic surfactant, 0 to 70 weight percent of solvent, and 1 to 20 weight percent of alkynol. The corrosion inhibitor has good effect of resisting the corrosion of the high-temperature high-pressure carbon dioxide, can form a layer of effective protective film on the surface of metal, prevent hypersalinity wastewater from corroding the metal at high temperature and high partial pressure of carbon dioxide, and can be used in oil wells with high temperature and high partial pressure of carbon dioxide.
Owner:HUAZHONG UNIV OF SCI & TECH +1
Hair conditioning composition comprising gel matrix and high molecular weight water-soluble cationic polymer
InactiveUS20060286059A1Improved conditioning benefitEasy to cleanCosmetic preparationsHair cosmeticsCompound (substance)Water soluble
Disclosed is a hair conditioning composition comprising by weight: (a) from about 0.01% to about 10% of a high molecular weight water-soluble cationic polymer having a molecular weight of about 5,000,000 or more; (b) a gel matrix comprising: from about 0.1% to about 10% by weight of the composition of a cationic surfactant where in the cationic surfactant is a mono-long alkyl quaternized ammonium; from about 4.5% to about 20% by weight of the composition of a high melting point fatty compound; and an aqueous carrier. The composition of the present invention can provide another benefit such as clean rinse feel during and after rinsing while maintaining conditioning benefits of the gel matrix, and / or provide improved conditioning benefits especially wet conditioning benefits while maintaining the above dry conditioning benefits.
Owner:THE PROCTER & GAMBLE COMPANY
Organic soil for oil base drilling fluids with high yield value and preparation method thereof
The invention relates to organic soil for oil base drilling fluids with a high yield value. The organic soil is formed by reacting montmorillonite, an alkyl quaternary ammonium salt surfactant, a quaternary ammonium salt cationic surfactant with a strong polar group and an anionic surfactant with a long chain alkyl group, wherein the input amount of the alkyl quaternary ammonium salt surfactant is 0.6-1.5 times of a sodium-based montmorillonite cation exchange capacity; the input amount of the quaternary ammonium salt cationic surfactant with the strong polar group is 0.01-0.5 times of the sodium-based montmorillonite cation exchange capacity; the molar ratio of the anionic surfactant with the long chain alkyl group to the quaternary ammonium salt cationic surfactant with the strong polar group is 1 to 1. According to the organic soil for oil base drilling fluids, the drilling fluids prepared by mineral oil has high yield value and can keep stable after being aged at high temperature.
Owner:ZHEJIANG FENGHONG NEW MATERIAL
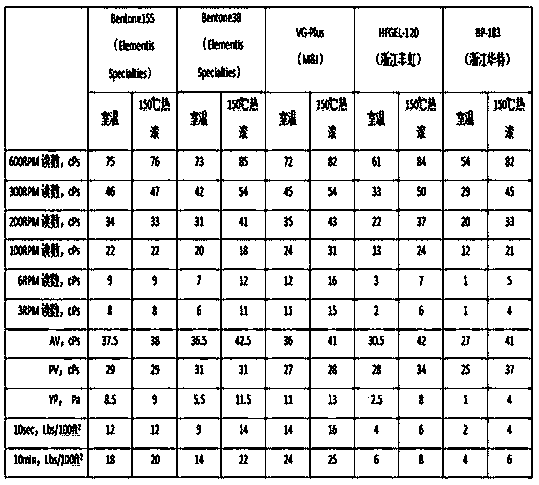
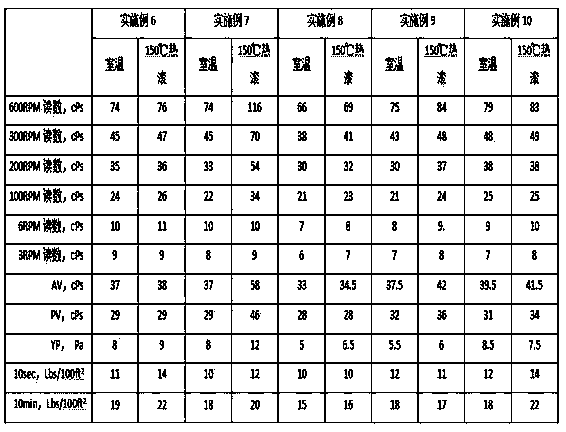
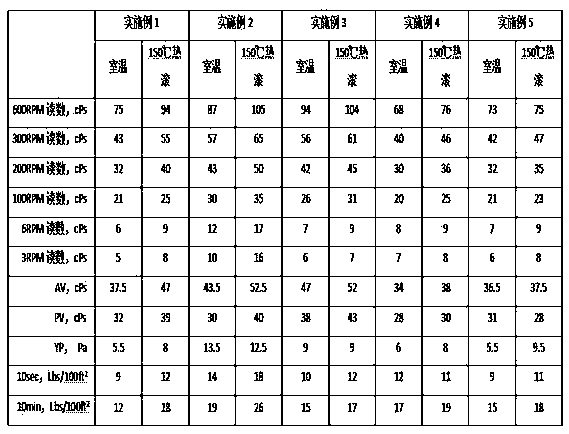
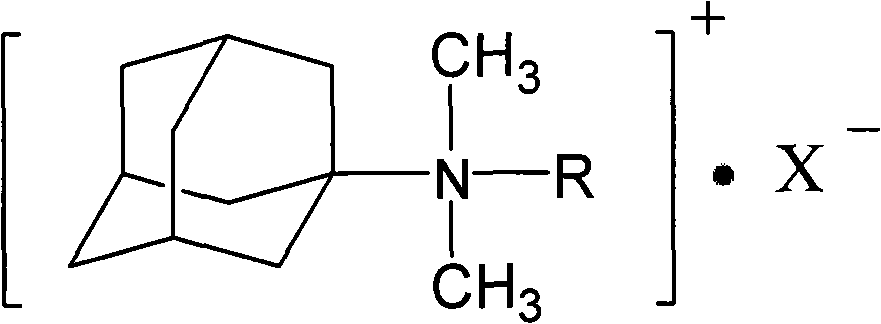
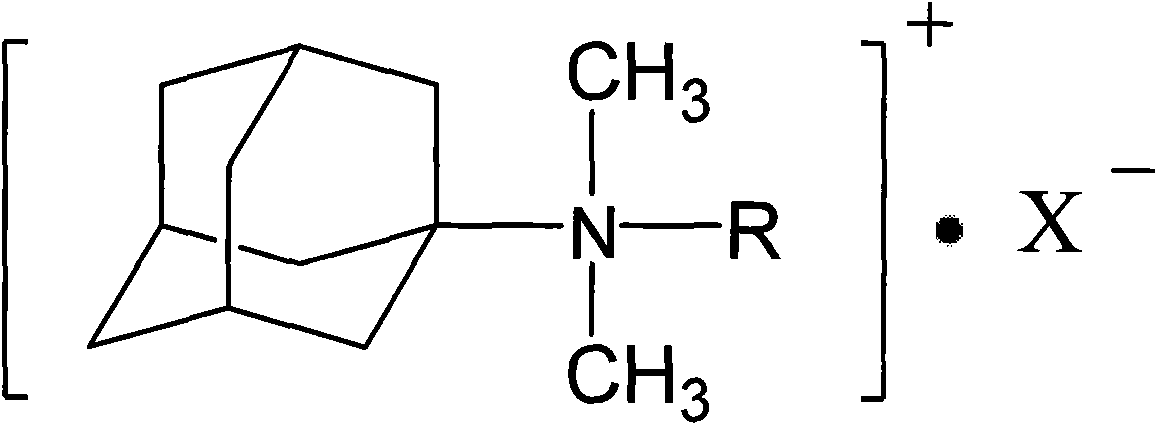
Adamantyl quaternary ammonium salt and preparation method thereof
InactiveCN101565375AHigh yieldHigh purityOrganic compound preparationPreparation by reductive alkylationSurface-active agentsStructural formula
The invention discloses an adamantyl quaternary ammonium salt and a preparation method thereof. The structural formula of the adamantyl quaternary ammonium salt is shown as right formula, wherein R represents phenmethyl or alkyl containing 1 to 18 carbon atoms; and X represents Cl, Br or I. The preparation method of the adamantyl quaternary ammonium salt adopts amantadine as a material and adopts two-step reaction as follows: adopting amantadine to synthesize adamantyl dimethyl-tert-amine and then synthesizing the adamantyl quaternary ammonium salt through quaterisation of the adamantyl dimethyl-tert-amine. The invention has easy operation, mild reaction process, high yield and purity and low product eco-toxicity. The cation surface active agent of the adamantyl quaternary ammonium salt can be potentially applied to high and new technology fields of micro-electronics, biomedicine, pharmacy, nanometer material and the like.
Owner:GUANGDONG UNIV OF TECH
Preparatino method of low-molecular chitosar quaternary ammonium salt complex iodine clear venom
InactiveCN1470167AImprove antibacterial propertiesMaintain concentrationBiocideAnimal repellantsQuaternary ammonium cationDisinfectant
The invention is a preparing method of the low-molecular chitosan quaternary-ammonium-salt complex iodine sterilizing solution, its character: mixing and agitating the low-molecular chitosan quaternary-ammonium-salt or low-molecular chitosan solution with one, two or all of dialkyl diquaterary ammonium salt, dialkyl quaterary ammonium salt, and methyl quaterary ammonium salt, then heating the mixed solution, adding with iodine, or iodine and iodate, preserving heat to react, and cooling to room temperature. It is an environmental-protection, strong-efficiency disinfectant, irritation and the toxicity lower, and the sterilizing effect more stable and permanent.
Owner:OCEAN UNIV OF CHINA
Attapulgite clay suspension agent
InactiveCN101987929AGood dispersionImprove suspension abilityCosmetic preparationsToilet preparationsSuspending AgentsSolvent
The invention discloses an attapulgite clay suspension agent, which mainly comprises attapulgite clay, other clays, chitosan and alkyl quaternary ammonium salt which serve as raw materials. The attapulgite clay suspension agent has the characteristics of high dispersibility and suspending power in water, has high dispersibility and suspension property in an ethanol solvent, and can be used as a suspended anti-settling agent, a thickening agent, a rheological aid and the like.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Cationic color fixing agent and preparation method and application thereof
The invention provides a cationic color fixing agent and a preparation method and application thereof. The cationic color fixing agent is obtained by polymerization reaction of an oil-soluble monomer, a cationic monomer and a reactive monomer in a solvent under the action of a radical initiator, wherein the oil-soluble monomer is one or the combination of several of styrene, acrylonitrile and acrylate; the cationic monomer is one or the combination of acryloxyethyl alkyl quaternary ammonium salt and / or allyl alkyl quaternary ammonium salt; the reactive monomer is one or the combination of several of allyl glycidyl ether, hydroxyl-containing acrylics, vinyl trimethoxy silane, vinyl triethoxy silane, acrylamide, crylic acid and methacrylic acid. The cationic color fixing agent is insoluble in water and can be dissolved in a weak polar solvent and / or non-polar solvent; besides, after non-aqueous solvent dyeing of reactive dyes, the cationic color fixing agent can be directly used in the on-going fixing process without washing.
Owner:GUANGDONG ESQUEL TEXTILES CO LTD
Non-aqueous electrolytes for electrical storage devices
InactiveUS6902684B1Improve conductivityGreat capacitanceHybrid capacitor electrolytesOrganic electrolyte cellsHydrogen fluorideSolvent
A non-aqueous electrolyte for electric storage devices consisting of a nitrile solvent and a complex salt formed by the reaction of a tetraalkyl ammonium salt and hydrogen fluoride. The electrolyte may include a component which a cation of an imidazolium or quaternary tetraalkylammonium salt.
Owner:LITHDYNE INTIOAL +1
Ultraviolet shielding agent containing MnO2 nano sheet and its preparing and using method
InactiveCN1944544AStrong UV shielding effectIncrease layer spacingCosmetic preparationsInorganic pigment treatmentPolyolefinManganese
The present invention belongs to the field of organic-inorganic composite material preparing technology, and is especially one new kind of ultraviolet shielding agent with nanometer MnO2 sheet and its preparation process and usage. The preparation process includes the first stripping laminated MnO2 and forming nanometer MnO2 sheet sol; and the subsequent self-assembling electrostatically with long chain alkyl quaternary ammonium salt cation to obtain the long chain alkyl quaternary ammonium salt cation-nanometer MnO2 sheet ultraviolet shielding agent. When the ultraviolet shielding agent is blended with polyolefin plastic through smelting, the long chain alkyl quaternary ammonium salt cation-nanometer MnO2 sheet with relatively great interval will be stripped under the action of high temperature and high shearing force and the nanometer MnO2 sheet will be distributed homogeneously in the plastic to exhibit excellent ultraviolet shielding performance.
Owner:BEIJING UNIV OF CHEM TECH
Composite drug carried microsphere, minocycline hydrochloride nano controlled-release composite drug carried microsphere system and preparation method thereof
InactiveCN101836961ALow toxicityGood slow releaseAntibacterial agentsTetracycline active ingredientsMicrosphereCholesterol
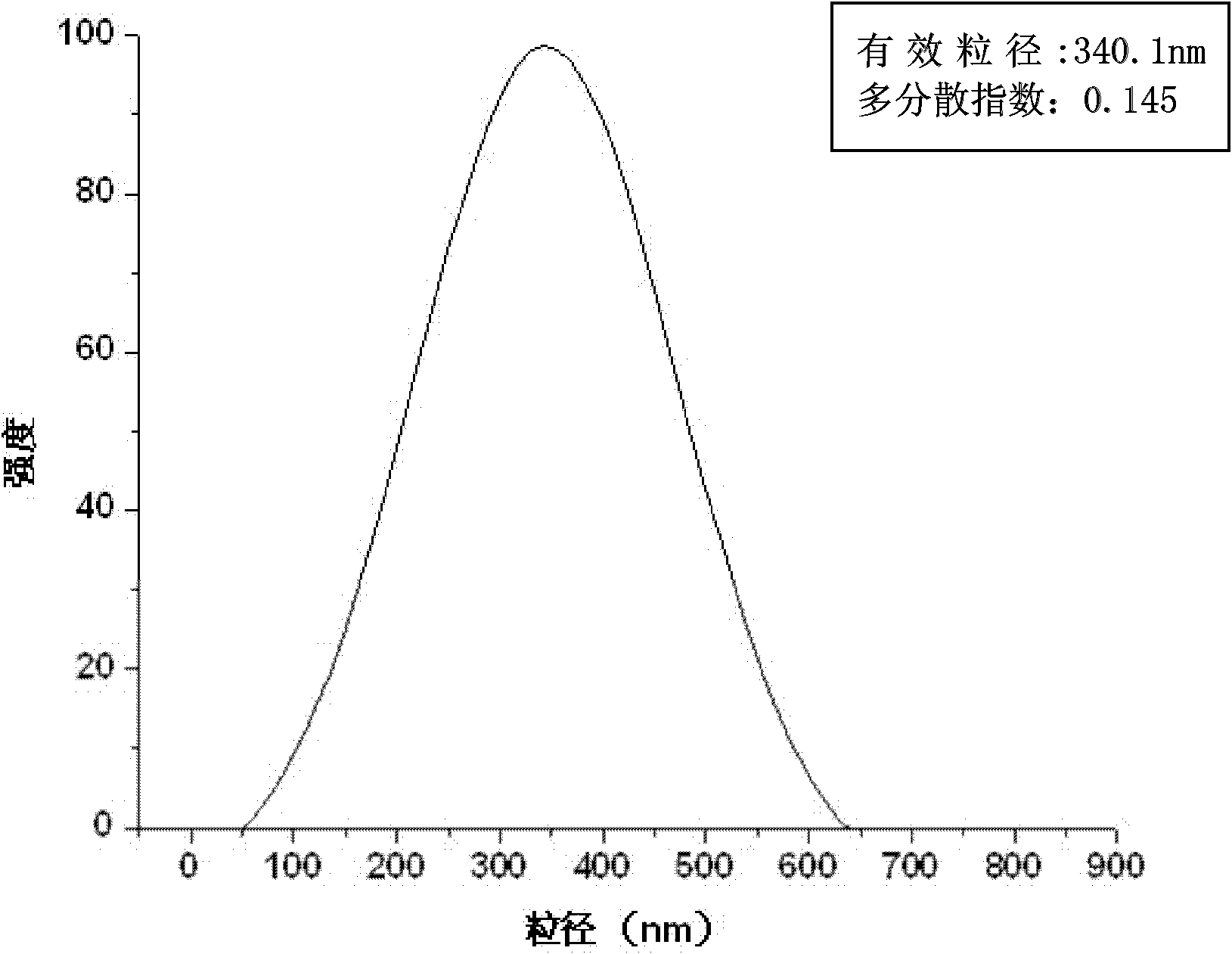
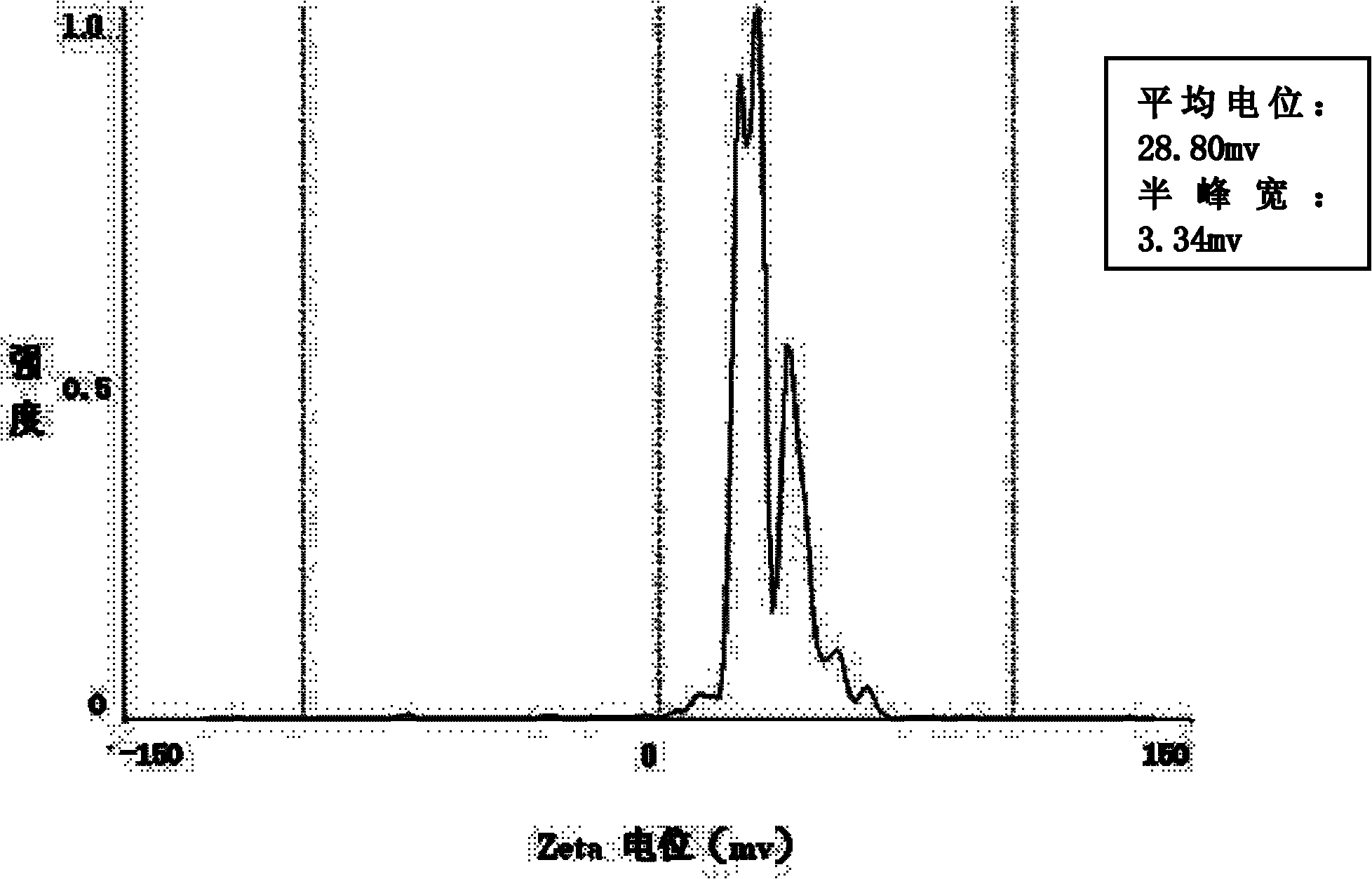
The invention relates to a composite drug carried microsphere, a minocycline hydrochloride nano controlled-release composite drug carried microsphere system and a preparation method thereof. A drug carried system with a nuclear shell structure is formed by embedding minocycline hydrochloride inside a poly D,L-lactide-co-glycolic acid polymer microsphere and covering a cationic polymeric liposome prepared from O-QACMC modified by polyethylene glycol, O-QACMC and cholesterol outside the poly D, L-lactide-co-glycolic acid polymer microsphere; and the composite drug carried microsphere system covered and carried with the minocycline hydrochloride has the grain diameter ranging from 340 nm to 400 nm and positive surface Zeta electric potential. The composite drug carried microsphere system can be remained in a water solution for at least 2 months, has high entrapment rate reaching larger than 90 percent on drugs and strong drug carrying capacity reaching 9 percent. The minocycline hydrochloride nano controlled-release composite drug carried microsphere system has the characteristics of uniform and controllable grain diameter, good preparation stability, simple preparation process, high drug carrying rate, favorable controlled release function, and the like, and is suitable for batch production.
Owner:TIANJIN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com