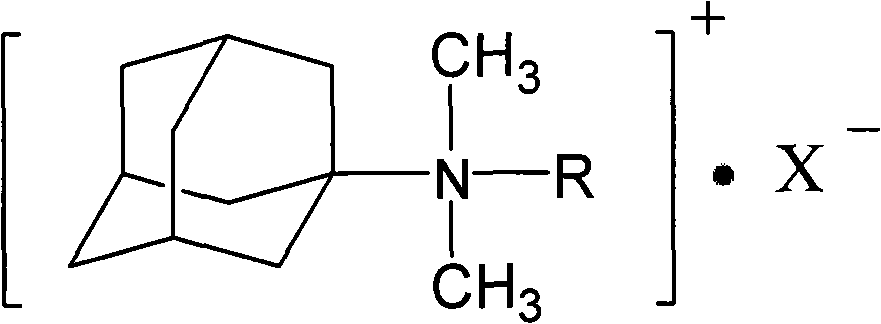
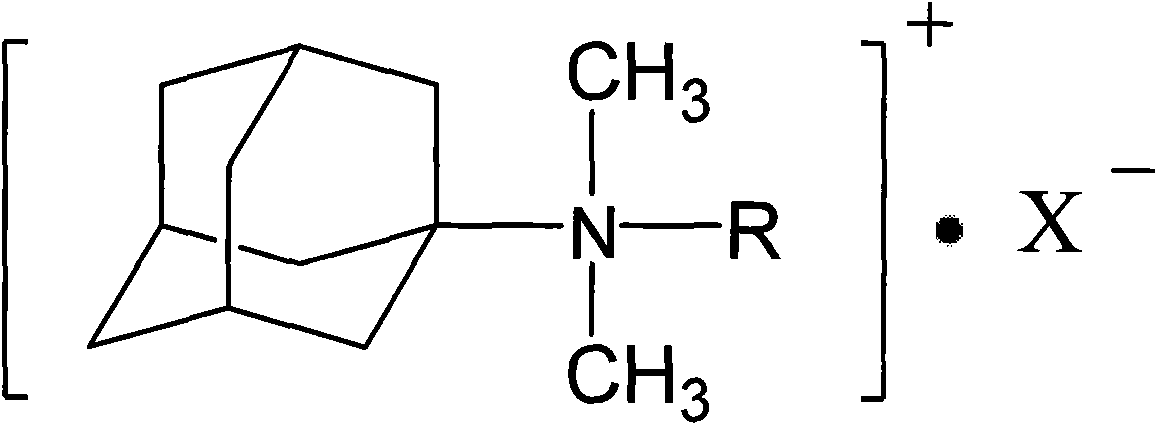
Adamantyl quaternary ammonium salt and preparation method thereof
A technology of adamantyl and quaternary ammonium salts, applied in the direction of reductive alkylation preparation, preparation of amino compounds, chemical instruments and methods, etc., can solve problems such as poor microbial effect, small antibacterial spectrum, and difficulty in large-scale production, and achieve Low ecotoxicity of the product, mild reaction conditions, simple and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 15.1g of amantadine and 189mL of methanol to a 500mL three-necked flask equipped with a reflux condenser, heat while stirring, first add 46g of formic acid at 10°C within 40min, and at 50°C, add 46g of formaldehyde within 4.5h; then raise the temperature To 70°C, react for 30h. After the reaction was completed, the sodium hydroxide solution was used to adjust the alkalinity, the upper solution was separated, and washed with water until the water layer was neutral, and the organic layer was vacuum-dried to obtain adamantyl dimethyl tertiary amine with a yield of 91%.
[0022] Put 15g of adamantyl dimethyl tertiary amine and 185mL of methanol into a 500mL three-necked flask, then add 53.1g of benzyl chloride, raise the temperature to 60°C and continue to stir for 20 hours, then distill off the solvent under reduced pressure, wash with excess ether, vacuum After drying, the corresponding adamantyl dimethyl benzyl quaternary ammonium salt was obtained with a yield of 97...
Embodiment 2
[0024] Add 15.1g of amantadine and 19mL of n-pentanol to a 100mL three-necked flask equipped with a reflux condenser, heat while stirring, add 9.2g of formic acid within 40 minutes at 20°C, and add 6g of formaldehyde within 4.5h at 65°C ; Then the temperature was raised to 120°C, and the reaction was carried out for 5h. After the reaction was completed, the sodium hydroxide solution was used to adjust the alkalinity, the upper layer solution was separated, and washed with water until the water layer was neutral, and the organic layer was vacuum-dried to obtain adamantyl dimethyl tertiary amine with a yield of 88%.
[0025] Put 15g of adamantyl dimethyl tertiary amine and 9.5mL of n-pentanol into a 100mL three-necked flask, then add 10.7g of benzyl chloride, then raise the temperature to 120°C and continue stirring for 3 hours, then distill off the solvent under reduced pressure, and use excess Washing with ether and drying in vacuo gave the corresponding adamantyl dimethyl ben...
Embodiment 3
[0027] Add 30g of amantadine and 200mL of isopropanol to a 500mL three-neck flask equipped with a reflux condenser, heat while stirring, first add 46g of formic acid within 40 minutes at 35°C, and add 24g of formaldehyde within 4.5h at 50°C; then Raise the temperature to 80°C and react for 21h. After the reaction was completed, the sodium hydroxide solution was used to adjust the alkalinity, the upper solution was separated, and washed with water until the water layer was neutral, and the organic layer was vacuum-dried to obtain adamantyl dimethyl tertiary amine with a yield of 92%.
[0028] Put 30g of adamantyl dimethyl tertiary amine and 190mL of isopropanol into a 500mL three-necked flask, then add 25.3g of benzyl chloride, then raise the temperature to 80°C and continue stirring for 9 hours, then distill off the solvent under reduced pressure, and use excess ether Washing, vacuum drying to obtain the corresponding adamantyl dimethyl benzyl quaternary ammonium salt, yield 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com