Amphiphilic chitosan quatermary ammonium salt with long alkane radical and its prepn
A long-chain alkyl and epoxy long-chain alkyl technology, which is applied in the field of amphiphilic chitosan long-chain alkyl quaternary ammonium salt and its preparation, can solve problems such as difficulty in dissolution, difficulty in research and application, and reduction in the scope of use. , to achieve high degree of substitution of quaternary ammonium salts, simple and easy preparation process, and simple and easy method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
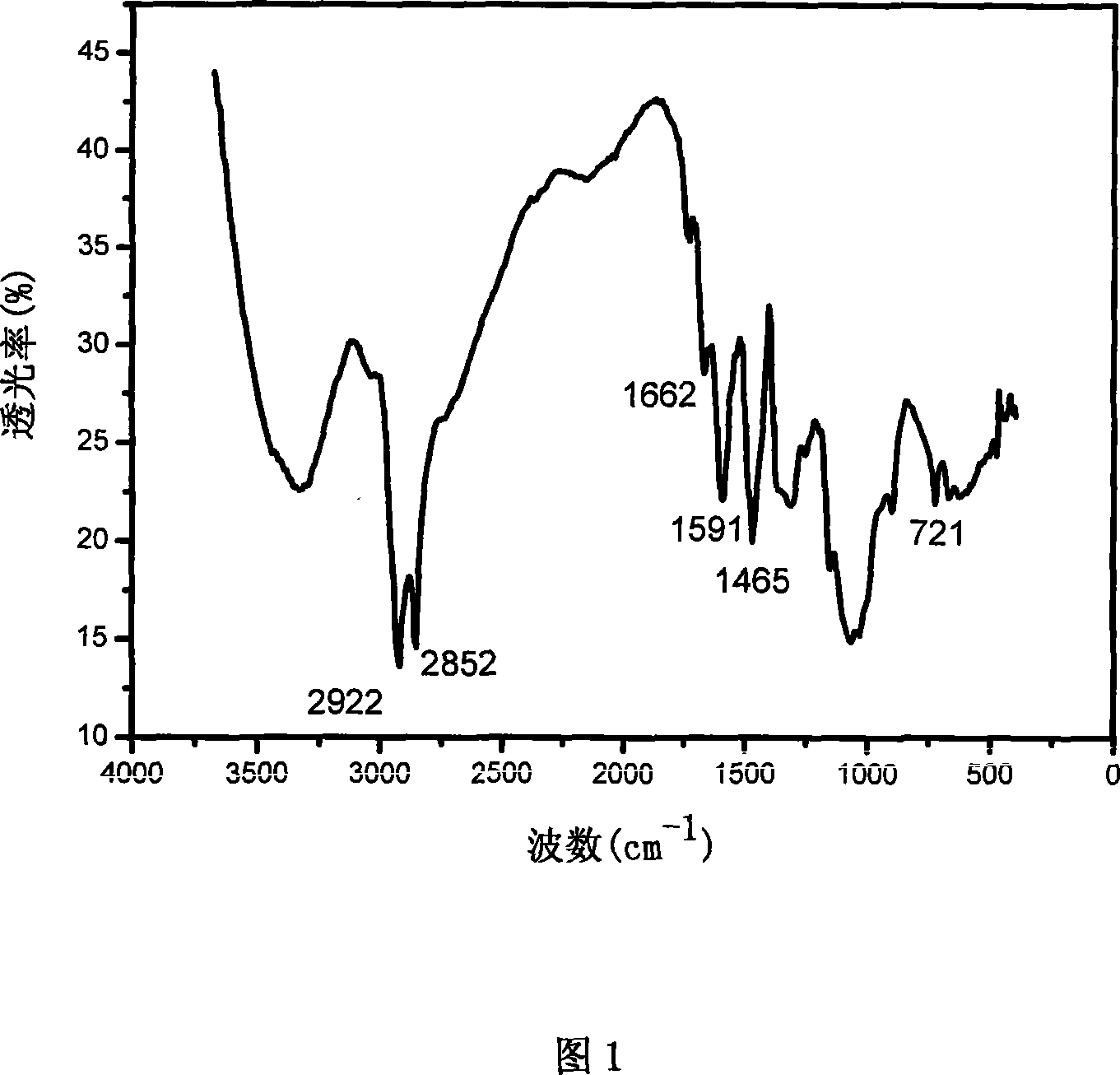
[0036] Put 12g of octadecyldimethyl tertiary amine in a four-necked flask, add 60ml of solvent, stir vigorously, raise the temperature to 55°C, slowly add 5.5g of epichlorohydrin dropwise, keep it under reflux for several hours, and distill under reduced pressure to remove unreacted Epichlorohydrin and solvent to obtain light yellow paste dimethyl epoxypropyl octadecyl ammonium chloride.
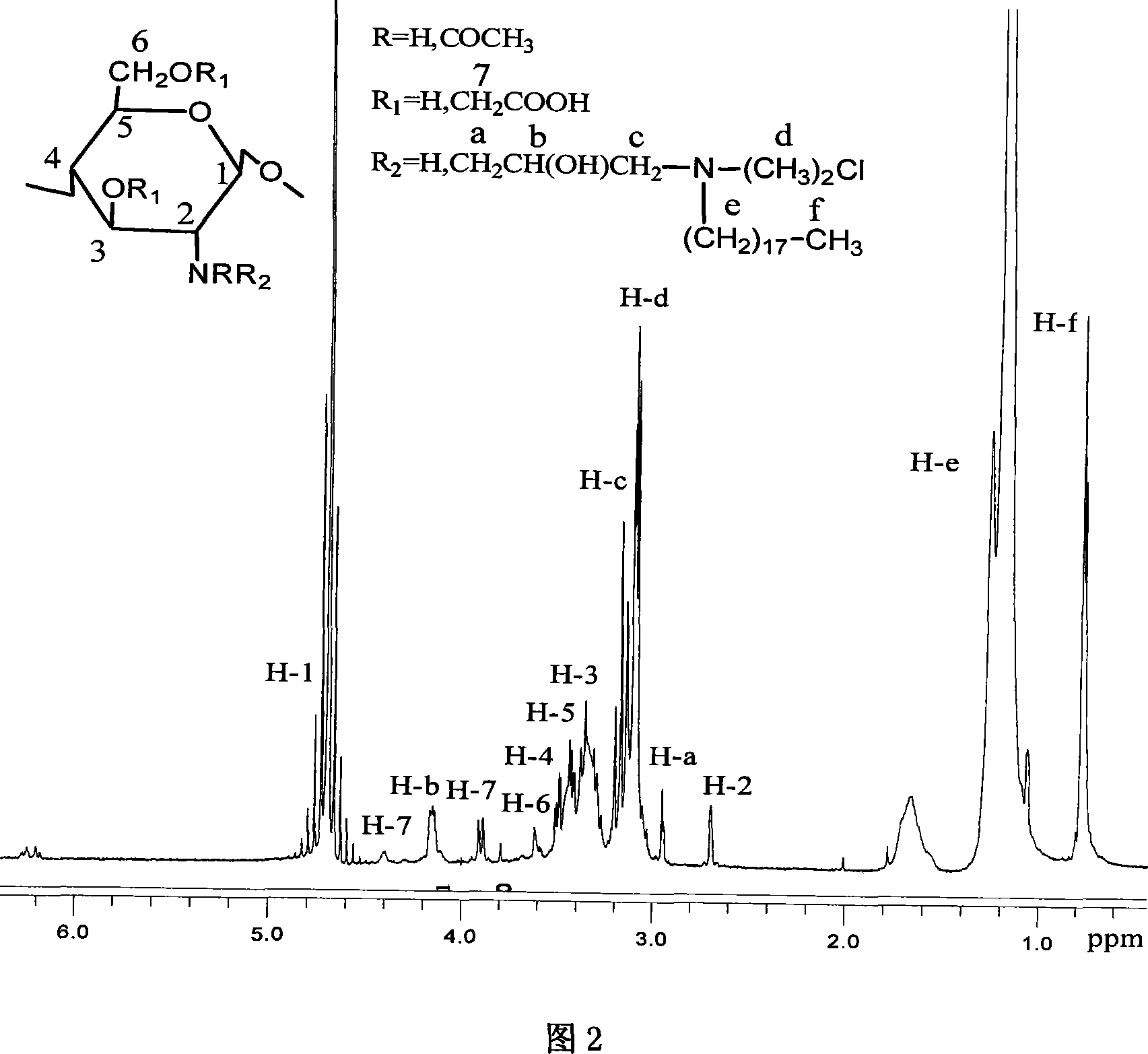
[0037] Get water-soluble carboxymethyl chitosan (viscosity-average molecular weight 100,000, deacetylation degree 80%) 3.0g is dissolved in the NaOH solution 100mL that concentration is 42% (w / v), after stirring evenly, add isopropanol 50mL, Slowly add 0.1 mol of dimethylglycidyl octadecylammonium chloride in batches, control the temperature at 80°C-85°C, and stir for 48h. Adjust the pH to 7 with hydrochloric acid, wash with anhydrous acetone, and dry in vacuum to obtain amphiphilic carboxymethyl chitosan octadecyl ammonium chloride.
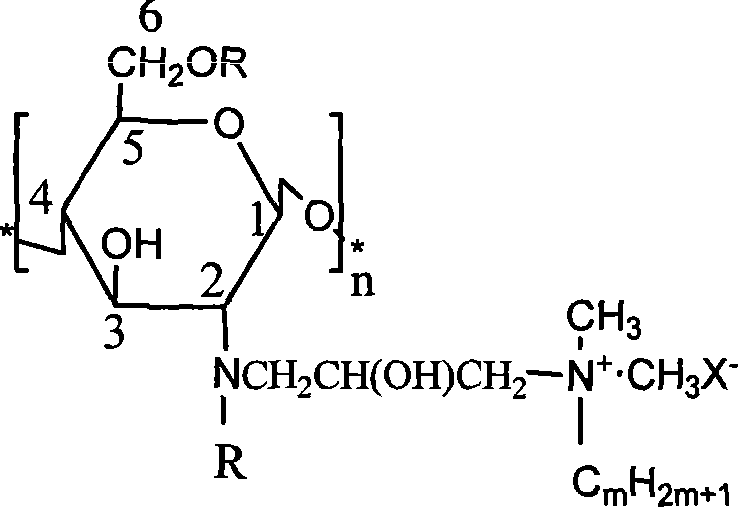
[0038] Gained chitosan derivatives are compounds of for...
Embodiment 2
[0044] Put 10g of octyl dimethyl tertiary amine in a four-neck flask, add 60ml of solvent, stir vigorously, raise the temperature to 55°C, slowly add 5.5g of epichlorohydrin dropwise, keep it under reflux for several hours, and distill under reduced pressure to remove unreacted Epoxychlorohydrin and solvent to obtain light yellow paste dimethyl epoxypropyl octyl ammonium chloride.
[0045] Get water-soluble lysine chitosan (viscosity-average molecular weight 50,000, degree of deacetylation 80%) 3.0g is dissolved in the NaOH solution 100mL that concentration is 42% (w / v), after stirring evenly, add isopropanol 50mL, Slowly add 0.1 mol of dimethylglycidyl octyl ammonium chloride in batches, control the temperature at 80°C-85°C, and stir for 48h. Adjust the pH to 7 with hydrochloric acid, wash with anhydrous acetone, and dry in vacuum to obtain amphiphilic lysine chitosan octyl ammonium chloride.
[0046] Gained chitosan derivatives are compounds of formula (2):
[0047]
[...
Embodiment 3
[0050] Put 11g of dodecyl dimethyl tertiary amine in a four-neck flask, add 60ml of solvent, stir vigorously, raise the temperature to 55°C, slowly add 5.5g of epichlorohydrin dropwise, keep warm and reflux for several hours, and distill under reduced pressure to remove unreacted Epichlorohydrin and solvent to obtain light yellow paste dimethyl epoxypropyl dodecyl ammonium chloride.
[0051] Take 3.0 g of water-soluble O-hydroxyethyl chitosan (viscosity-average molecular weight 700,000, deacetylation degree 80%) and dissolve it in 100 mL of NaOH solution with a concentration of 42% (w / v). After stirring evenly, add isopropanol 50 mL, slowly add 0.1 mol of dimethylglycidyl dodecyl ammonium chloride in batches, control the temperature at 80°C-85°C, and stir for 48h. Adjust the pH to 7 with hydrochloric acid, wash with anhydrous acetone, and dry in vacuum to obtain amphiphilic O-hydroxyethyl chitosan dodecyl ammonium chloride.
[0052] Gained chitosan derivatives are compounds o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com