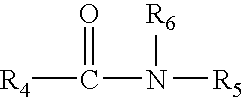
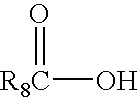
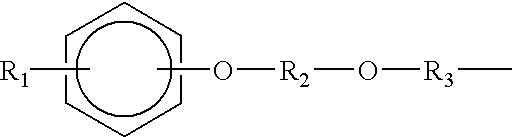Transdermal compositions
a technology of compositions and transdermal layers, applied in the field of transdermal compositions, can solve the problems of imposing cost, multiplicity of problems, and difficulty in designing compositions for transdermal use, and achieve the effect of meeting all of these requirements for all skin conditions and reducing the difficulty of preparing a wide range of effective compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Formation of Distearyl Stearamide and Cetyl Glycerol Laurate
[0236] An equimolar mixture of stearic acid and dimethyl distearyl ammonium chloride (DDAC) was dissolved in water in the presence of triethanolamine (TEA) wherein the TEA was present such that the mole ratio of DDAC to TEA was 10:1. The aqueous mixture was maintained at a temperature in excess of 60.degree. C. and at a pH in the range of from about 3 to about 5.
[0237] The reaction proceeded with the evolution of gas. The pH of the reaction mixture was measured during this reaction. The pH dropped exponentially from a pH in the range of 5 to 6 down to a pH of 2 to 3 within 5 minutes. This is quite remarkable insofar as the pH of stearic acid in water is about 4.5 and the pH of DDAC is about 6.
[0238] Cetyl alcohol and glycerol monolaurate were thereupon added to the reaction mixture in a concentration of 4 moles of each of these compounds per mole of DDAC. Gellation and subsequent creaminess occurred. The reaction mixture wa...
example 2b
Formation of DDAS and Triethanolamine Stearate (TEAS)
example 2a
[0243] repeated but for the sequence of addition of glycerol monolaurate and cetyl alcohol. Instead of being added in the second step, after the equimolar mixture of stearic acid and DDAC, the same amount of the glycerol monolaurate and cetyl alcohol added in Example 2 was added after the addition of TEA.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight ratio | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com