Parecoxib sodium pharmaceutical composition for injection
A technology of parecoxib sodium and parecoxib, which is applied in the direction of drug combinations, active ingredients of heterocyclic compounds, antipyretics, etc., can solve problems such as inconvenience in the production process, achieve stable quality, simple and easy method, and reduce The effect of market risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
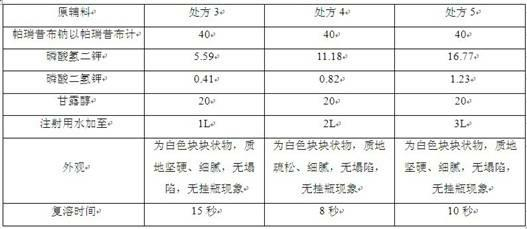
[0081] The formula of every 1000 described parecoxib sodium pharmaceutical compositions consists of:
[0082] Parecoxib sodium as parecoxib 40g
[0083] Dipotassium hydrogen phosphate 11.18g
[0084] Potassium dihydrogen phosphate 0.82g
[0085] Mannitol 20g
[0086] Appropriate amount of sodium hydroxide
[0087] Add water for injection to 2ml.
[0088] The preparation process includes:
[0089] 1) Weigh dipotassium hydrogen phosphate and potassium dihydrogen phosphate, add appropriate amount of water for injection to dissolve until completely, and set aside;
[0090] 2) Take 90% of the phosphate buffer solution in 1), and the temperature is 40±5°C, add the prescribed amount of parecoxib sodium, and stir until it is completely dissolved by visual inspection;
[0091] 3) Add 0.1% medical activated carbon to the solution in 2) and stir for 15 minutes to decolorize. After decolorization, filter and remove the carbon, add an appropriate amount of water ...
Embodiment 2
[0099] The formula of every 1000 described parecoxib sodium pharmaceutical compositions consists of:
[0100] Parecoxib sodium as parecoxib 20g
[0101] Dipotassium hydrogen phosphate 11.18g
[0102] Potassium dihydrogen phosphate 0.82g
[0103] Mannitol 40g
[0104] Appropriate amount of sodium hydroxide
[0105] Add water for injection to 2ml.
[0106]Preparation process: with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com