Levocarnitine pharmaceutical composition for injection
A composition and a technique for injection, which are applied in the field of pharmaceuticals in the field of medicine, can solve problems such as inconvenience in the production process, and achieve the effects of stable quality, good stability, and simple and easy methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
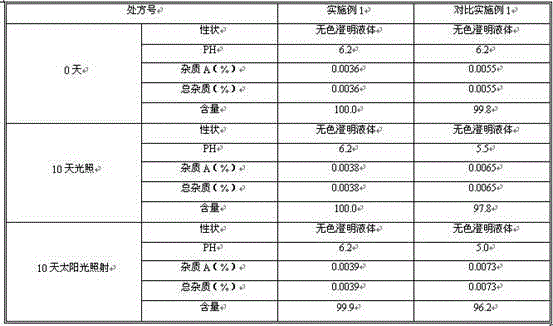
Embodiment 1
[0068] For every 1000 pieces of the L-carnitine pharmaceutical composition, the formula is as follows:
[0069] L-Carnitine 1000g
[0070] Citrate buffer (pH=6.2) 2.5L
[0072] The water for injection is increased to 5L.
[0073] Preparation Process:
[0074] 1) Weigh sodium chloride, add citrate buffer solution, and add appropriate amount of water for injection to dissolve it completely;
[0075] 2) Add 0.1% medicinal charcoal of the volume of water for injection, heat for 5 minutes to remove pyrogen, filter and decarbonize to obtain filtrate;
[0076] 3) Cool to 40-50℃, then add L-carnitine, dissolve it, add water for injection to the full amount, if necessary, adjust the pH range 5.5-7.0 with citric acid solution or sodium hydroxide solution;
[0077] 4) The resulting solution is finely filtered with a mixed cellulose ester microporous filter membrane to obtain the filtrate, which is filled and sealed in an ampoule, and autoclaved at 121°C for 15 minutes to o...
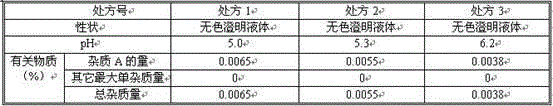
Embodiment 2
[0079] For every 1000 pieces of the L-carnitine pharmaceutical composition, the formula is as follows:
[0080] L-Carnitine 1000g
[0081] Citrate buffer (pH=6.2) 3L
[0082] Sodium chloride 30g
[0083] The water for injection is increased to 5L.
[0084] Preparation process: the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com