Solution-type metered dose inhalation aerosol for treating respiratory diseases and preparation method thereof
A technology of quantitative inhalation and aerosol, which is applied in the field of solution-type quantitative inhalation aerosol for the treatment of respiratory diseases and its preparation and inhalation aerosol. In order to achieve the effect of anti-airway inflammation and good bronchiectasis
Inactive Publication Date: 2014-05-14
BEIJING FSWELCOME TECH DEV +1
View PDF18 Cites 5 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, the fine particle fraction (Fine Particle Fraction, FPF) value of glycopyrronium bromide suspension inhalation is low, resulting in a decrease in the effective dose into the lungs, and there are many other problems in the suspension type metered inhalation aerosol, For example: (1) The water content should be controlled to be extremely low to avoid the adhesion and aggregation of drug particles; (2) The smaller the solubility of the drug in the propellant, the better, so as to avoid the coarsening of the drug crystallites during storage; (3) The particle size of the drug should be It must be less than 5 μm and not more than 10 μm, which is necessary for strengthening, preventing clogging of valves and maintaining valve sealing performance; (4) Appropriate excipients such as surfactants with low HLB values must be added to play Wetting, Dispersion and lubrication, etc., to prevent drug coagulation and recrystallization; (5) It is necessary to adjust the density of the propellant and the suspended solid, and try to make the two equal, which is not conducive to the use of a single propellant
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0048]
[0049] After dissolving glycopyrronium bromide, Cremophor EL and ethanol, the product is filled with HFA-134a in two steps.
Embodiment 2
[0051]
[0052] After dissolving glycopyrronium bromide, ethanol and oleic acid, it is prepared by one-step filling with HFA-134a.
Embodiment 3
[0054]
[0055] After dissolving glycopyrronium bromide, PEG1000, soybean lecithin and ethanol, it is obtained by one-step filling and filling with HFA-134a.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Login to View More
Abstract
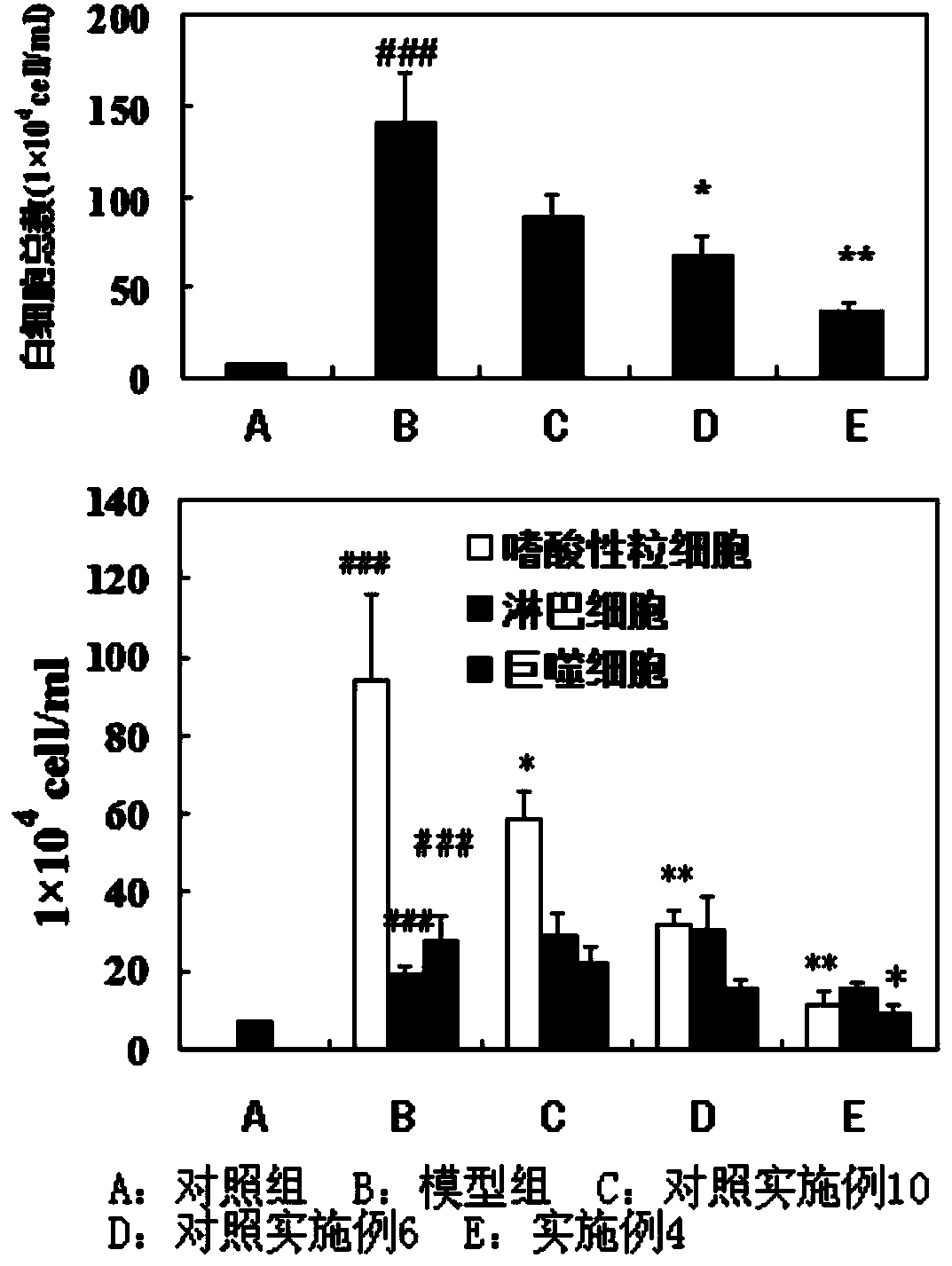
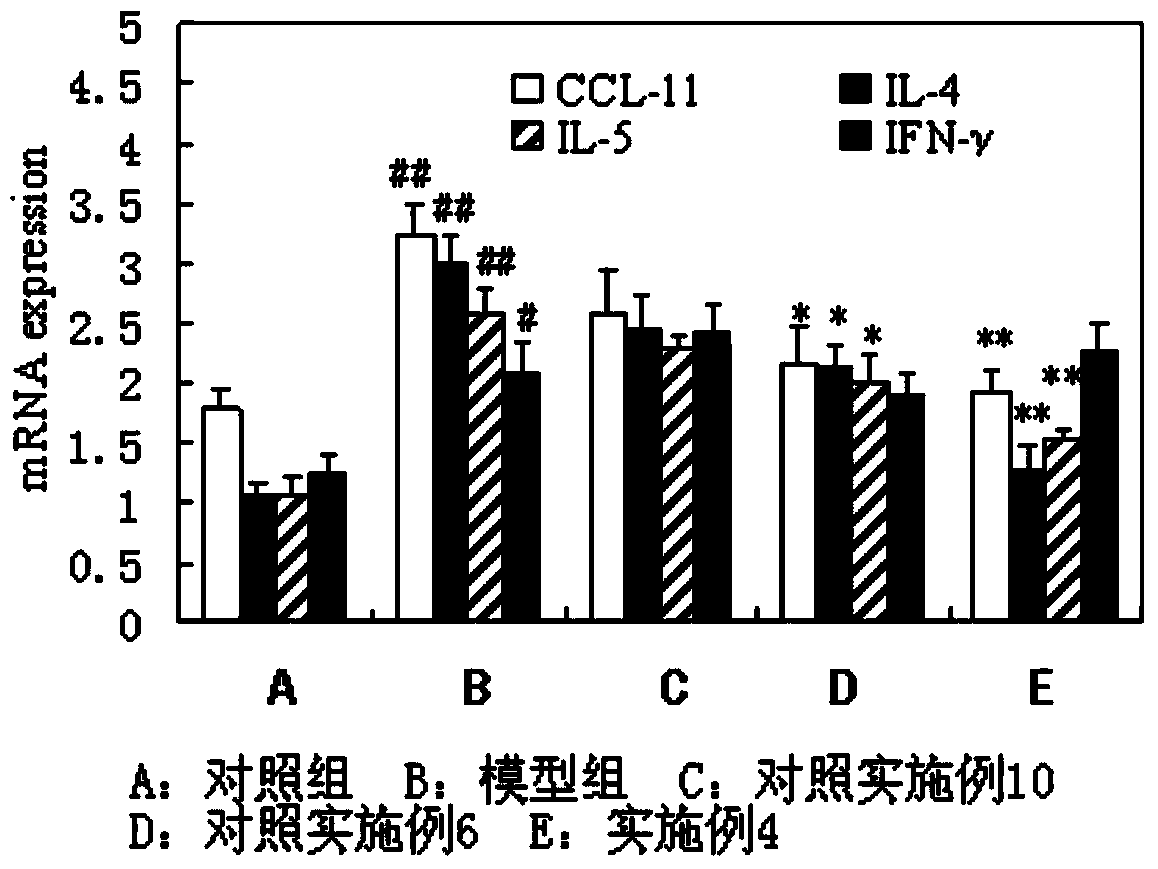
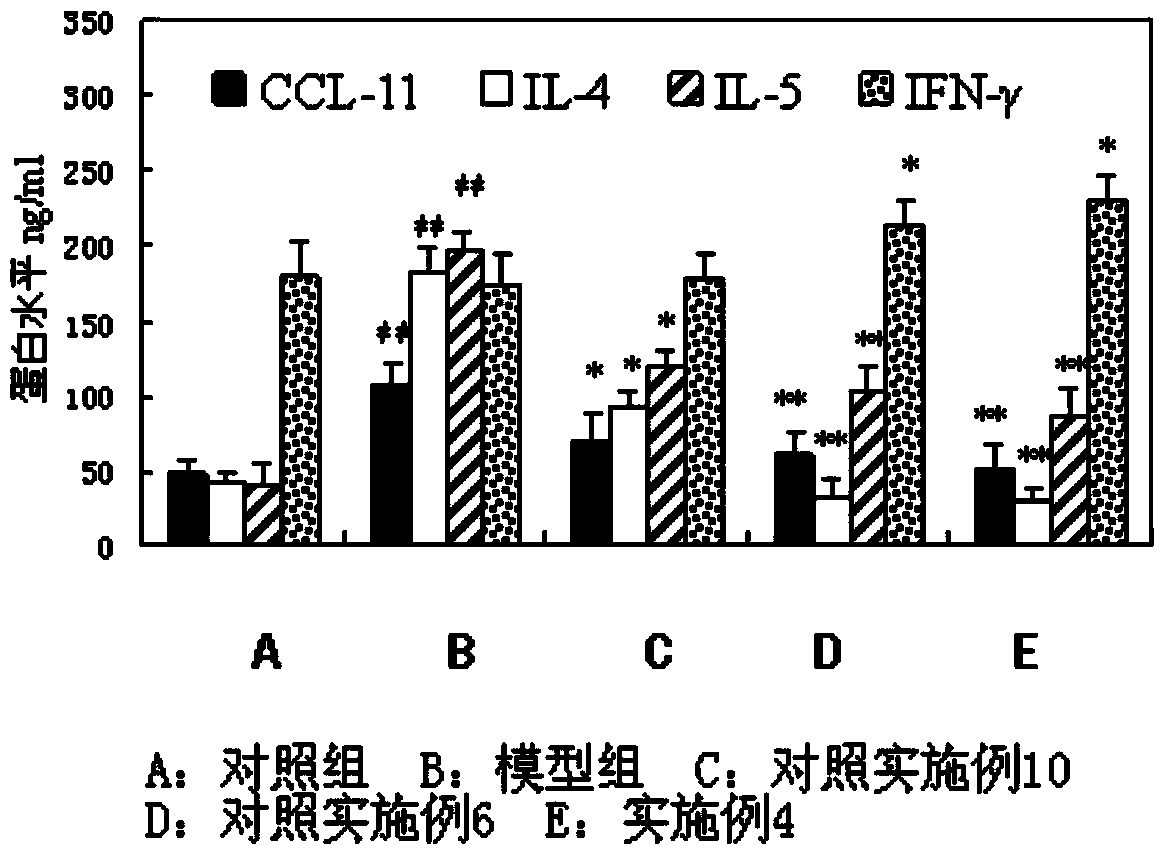
The invention discloses a solution type metered dose inhalation aerosol of glycopyrronium bromide or derivatives thereof and a preparation method thereof. The aerosol comprises a main drug, a cosolvent, a surfactant and a propellant. The aerosol is high in fine particle fraction (FPF) and good in placement stability under long-term reserving and accelerating conditions, and the FPF does not have remarkable variation. Pharmacodynamic test results indicate that the aerosol is quick in response, can be used for treating bronchial asthma and chronic obstructive pulmonary disease, and has excellent effects of resisting airway inflammation, airway fibrosis and airway remodeling.
Description
technical field [0001] The invention relates to an inhalation aerosol in the technical field of pharmaceutical preparations, in particular to a solution-type quantitative inhalation aerosol for treating respiratory diseases and a preparation method thereof. Background technique [0002] Since the invention of pressure metered dose inhaler (pressurized metered dose inhaler, pMDI) by Charles Thiel of Riker Laboratories in 1956, in the past 50 years, due to its rapidity, positioning and avoiding the first-pass effect of the gastrointestinal tract, as well as small size, It is widely used due to the convenience of drug administration, low price, easy use by patients, and no disadvantages such as dry powder inhaler loading drugs before use or moisture absorption of the powder in the device due to factors such as the environment. With the enhancement of people's awareness of environmental protection, in 1987, 40 countries signed the "Montreal Protocol on Substances that Deplete th...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K9/12A61K31/40A61K45/06A61K45/00A61P11/00A61P11/06
Inventor 陈小平吴予津谢诒诚高泽军
Owner BEIJING FSWELCOME TECH DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com