Aerosol preparation and quantitative inhalation aerosol
An aerosol and preparation technology, which is used in aerosol delivery, inhalers, respiratory diseases, etc., can solve problems such as the inability of drugs to enter the lungs, the decrease in the dose of fine particles, and the adverse effects of active ingredients in aerosols. Conducive to environmental protection, guaranteed curative effect, small damage effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] An aerosol preparation and a metered dose inhalation aerosol, comprising an active ingredient and a liquefied propellant as a solvent for the active ingredient, the active ingredient comprising budesonide and formolo fumarate, the liquefied propellant being hydrogen The fluorocarbon also includes PVPk25 and PEG1000 added to the liquefied propellant, the content of the PVPk25 in the aerosol preparation is 0.00005-0003wt%, and the content of the PEG1000 in the aerosol preparation is 0.001-0.01wt%.
[0028]In this embodiment, the above combination of budesonide and formoterol fumarate can be used as the treatment of respiratory diseases, that is, it is the active ingredient in this aerosol preparation, the above PVPk25 is the suspending agent in the aerosol preparation, and the above PEG1000 is a lubricant in aerosol formulations. Among the above components, by limiting the content of PVPk25 and PEG1000 in the aerosol preparation, the dosage of fine particles when the aero...
Embodiment 2
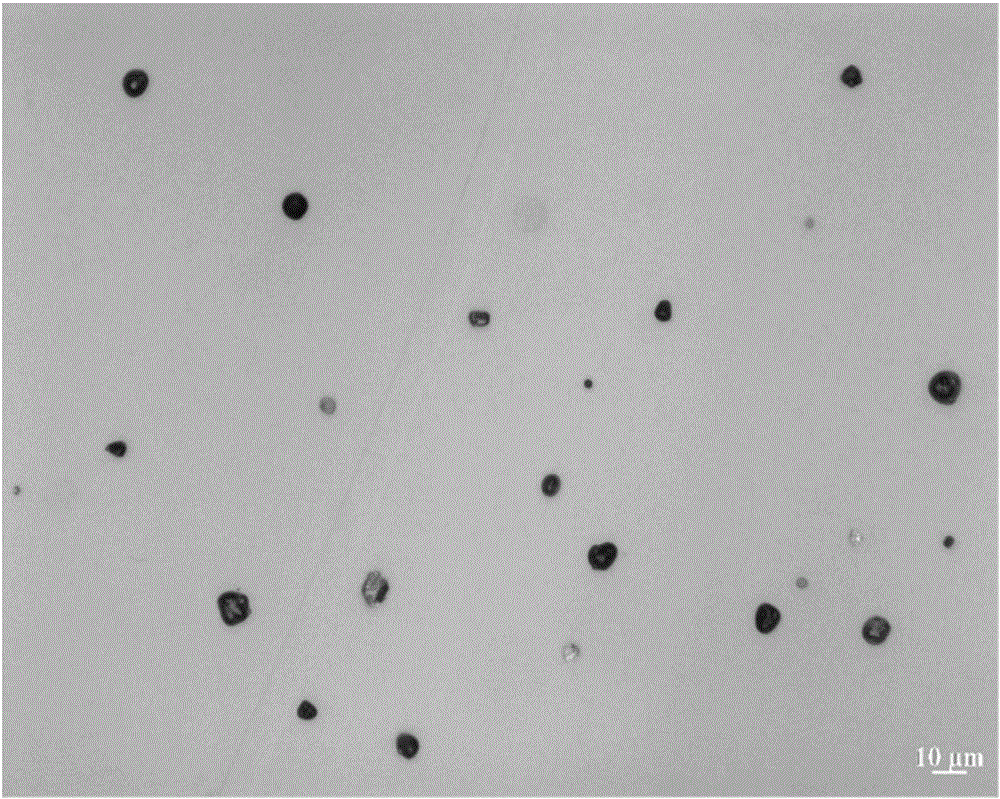
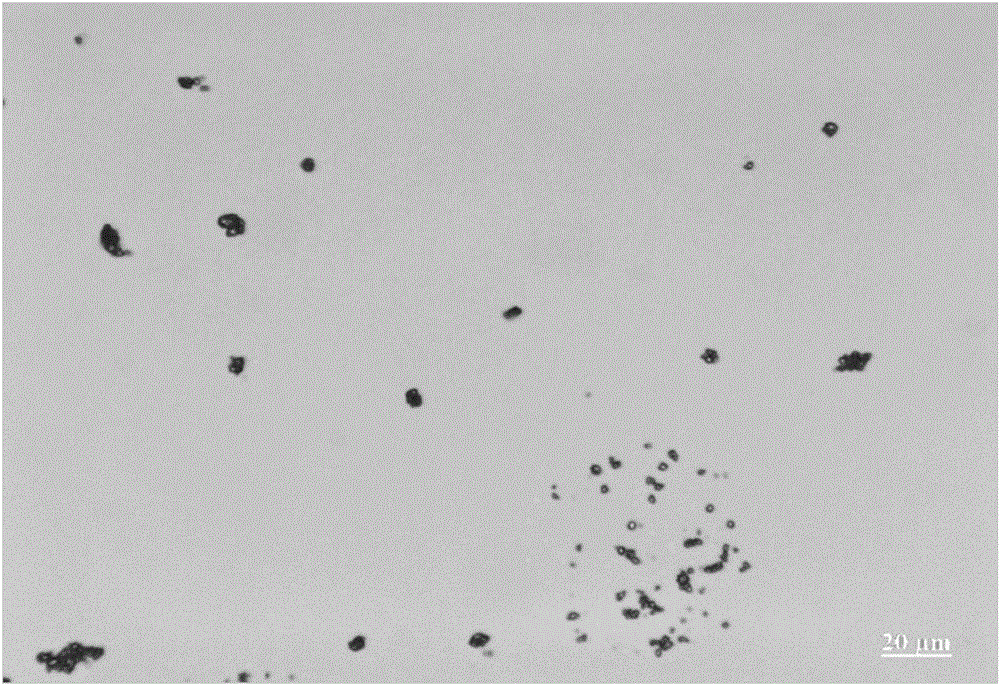
[0039] This example is further limited on the basis of Example 1, the content of the PVPk25 in the aerosol preparation is 0.0002wt%, and the content of the PEG1000 in the aerosol preparation is 0.0025wt%. In this scheme, the method for measuring the fine particle dose and delivery dose uniformity of each prescription is determined with reference to the method of "Chinese Pharmacopoeia" 2015 edition general rules (inhalation aerosol) 0111, and the devices used are as follows: delivery dose uniformity determination device, fine particle dose determination The device is used to measure, and the results obtained are: the dose of fine particles of the budesonide component can be about 60%, the dose of fine particles of the formoterol fumarate component can be about 67%, and the delivery dose uniformity of the budesonide component is relatively The standard deviation value is about 4.8%, and the relative standard deviation value of the delivery dose uniformity of the formoterol fumar...
Embodiment 3
[0041] This example is further limited on the basis of Example 1, the content of the PVPk25 in the aerosol preparation is 0.0002-0.001wt%. In this scheme, the method for measuring the fine particle dose and delivery dose uniformity of each prescription is determined with reference to the method of "Chinese Pharmacopoeia" 2015 edition general rules (inhalation aerosol) 0111, and the devices used are as follows: delivery dose uniformity determination device, fine particle dose determination The device is used for measurement, and the results obtained are: the dose of fine particles of the budesonide component is about 60%, the dose of fine particles of the formoterol fumarate component is about 68%, and the delivery dose uniformity of the budesonide component is relatively The standard deviation value is about 4%, and the relative standard deviation value of the delivery dose uniformity of the formoterol fumarate component is about 3.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com