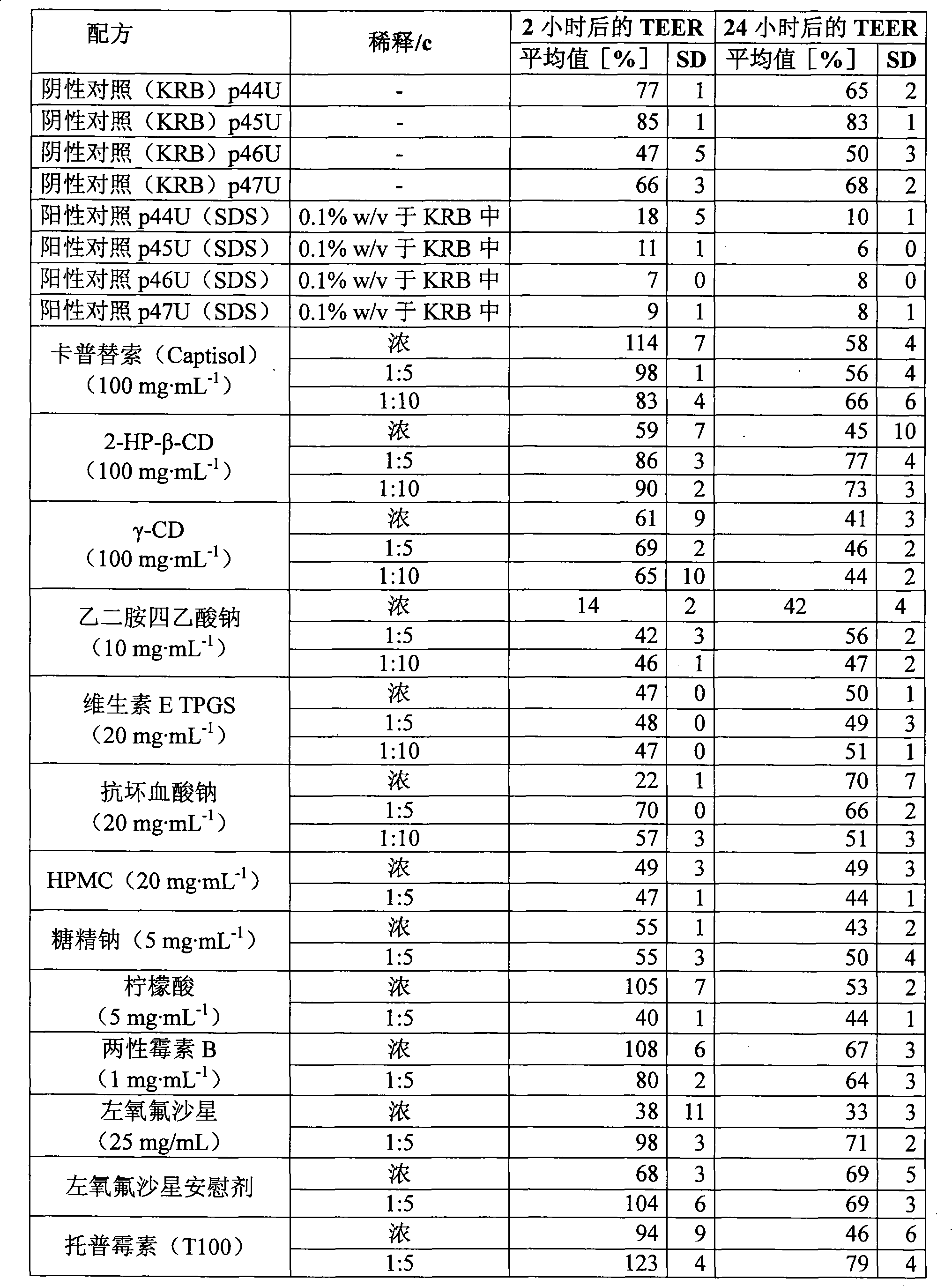
Nebulised antibiotics for inhalation therapy
A technology of antibiotics and aerosols, applied in the directions of anti-inflammatory agents, antibacterial agents, antiviral agents, etc., can solve the problems of difficult to manufacture in aseptic form, increased liquid volume, and difficult to effectively associate with water-soluble active compounds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0121] Dissolve levofloxacin hemihydrate (2.5g), chitosan (0.06g), hydroxypropyl methylcellulose (0.20g), magnesium sulfate hexahydrate (0.13g) and sodium chloride (0.71g) in water for injection , with a total weight of 100.0g. The solution was sterile filtered using 0.22 [mu]m filter paper and each 1.5 ml was aseptically filled into 2 ml preformed sterile blow-fill-seal vials in a laminar airflow hood, after which the vials were heat-sealed.
[0122] The liquid composition has a dynamic viscosity of 2.1 mPas. The surface tension is 47.8 mN / m. The pH value was 6.73 at 22°C and the osmolarity was 332 mOsmol / kg.
example 2
[0124] Levofloxacin hemihydrate (3.5g), 2-HP-βcyclodextrin (5g), magnesium sulfate hexahydrate (0.15g) and sodium chloride (0.25g) were dissolved in water for injection to a total weight of 100g. The resulting solution was sterile filtered using 0.22 μm filter paper, and 10 ml was aseptically filled into 15 ml glass vials. The solution has an osmolarity of 292 mOsmol / kg.
example 3
[0126] Dissolve levofloxacin hemihydrate (4g), tobramycin (6g), 2-HP-β cyclodextrin (10g), magnesium sulfate hexahydrate (0.15g) and sodium chloride (0.25g) in water for injection, The total weight reaches 100g. 1 ml each was filled into 2 ml preformed blow-fill-seal vials under aseptic conditions in a laminar airflow hood, after which the vials were heat-sealed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com