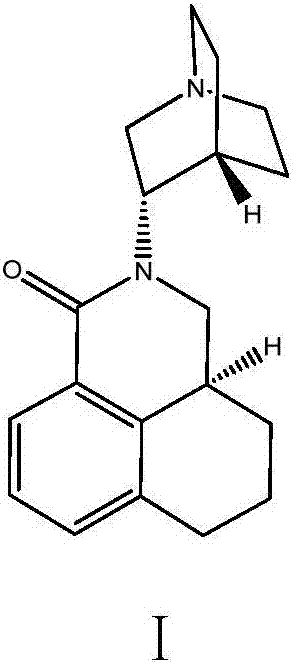
Solid medicine composition comprising palonosetron
The technology of palonosetron and composition is applied in the field of oral pharmaceutical composition containing 5-HT3 receptor antagonist and the field of preparation thereof, which can solve the problems of complicated prescription and the like, and achieves fast dissolution speed, good stability, and high performance. fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation of embodiment 1 palonosetron hard capsule
[0028]
[0029] Take the prescribed amount of palonosetron hydrochloride, add 5 times the amount of lactose and mix it, then carry out jet milling, then add 5 times the lactose to the obtained mixed powder and mix evenly, then add the remaining lactose and the prescribed amount of microcrystalline cellulose, carboxymethyl Add sodium starch and mix well, and finally add magnesium stearate and mix well. The obtained powder was sampled at 10 different positions, and an appropriate amount of powder (equivalent to 0.5 mg palonosetron) was weighed at each sampling point, and the moisture content was measured to determine the percentage content of each point. Capsule filling, inspection and packaging are carried out according to the detection results of the intermediate content, and the product is obtained.
Embodiment 2
[0030] The preparation of embodiment 2 palonosetron hard capsules
[0031]
[0032] Take the prescribed amount of palonosetron hydrochloride, add 5 times the amount of lactose and mix it, then carry out jet milling, then add 5 times the lactose to the obtained mixed powder and mix evenly, then add the remaining lactose and the prescribed amount of microcrystalline cellulose, carboxymethyl Add sodium starch and mix well, and finally add magnesium stearate and mix well. The obtained powder was sampled at 10 different positions, and an appropriate amount of powder (equivalent to 0.25 mg palonosetron) was weighed at each sampling point, and the moisture content was measured to determine the percentage content of each point. Capsule filling, inspection and packaging are carried out according to the detection results of the intermediate content, and the product is obtained.
Embodiment 3
[0033] The preparation of embodiment 3 palonosetron hard capsules
[0034]
[0035] Take the prescribed amount of palonosetron hydrochloride, add 5 times the amount of lactose and mix it, then carry out jet milling, then add 5 times the lactose to the obtained mixed powder and mix evenly, then add the remaining lactose and the prescribed amount of microcrystalline cellulose, carboxymethyl Add sodium starch and mix well, and finally add magnesium stearate and mix well. The obtained powder was sampled at 10 different positions, and an appropriate amount of powder (equivalent to 0.75 mg palonosetron) was weighed at each sampling point, and the moisture content was measured to determine the percentage content of each point. Capsule filling, inspection and packaging are carried out according to the detection results of the intermediate content, and the product is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com