Method for synthesizing palonosetron metabolite
A technology of palonosetron and metabolites, which is applied in the field of medicine, can solve the problems of complex operation, high cost, and long steps, and achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
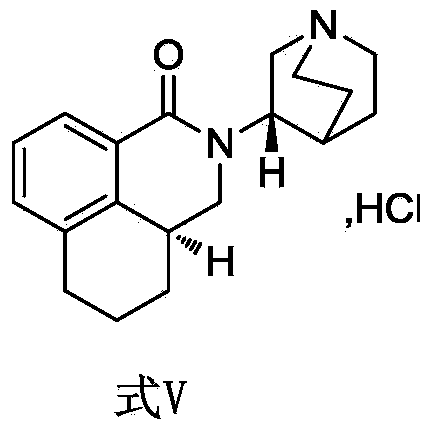
[0054] In a 250ml three-necked reaction flask, add (3aS,3'S)-2-[-1-azabicyclo[2.2.2]oct-3-yl]-2,3,3a,4,5,6-hexahydro- 1-Oxygen-1H-benzo [de] isoquinoline 5g (0.017mol), 25ml dichloromethane, then under stirring condition, drop 2.5g (0.029g) m-chloroperoxybenzoic acid dissolved in 25ml dichloromethane mol); after the addition is complete, react at 25°C for 2 hours, and take samples for monitoring.
[0055] Post-processing: stop the reaction, filter, wash twice with saturated sodium carbonate, dry over anhydrous magnesium sulfate, filter, and concentrate to dryness to obtain 4.8 g of off-white solid with a yield of 91.1%.
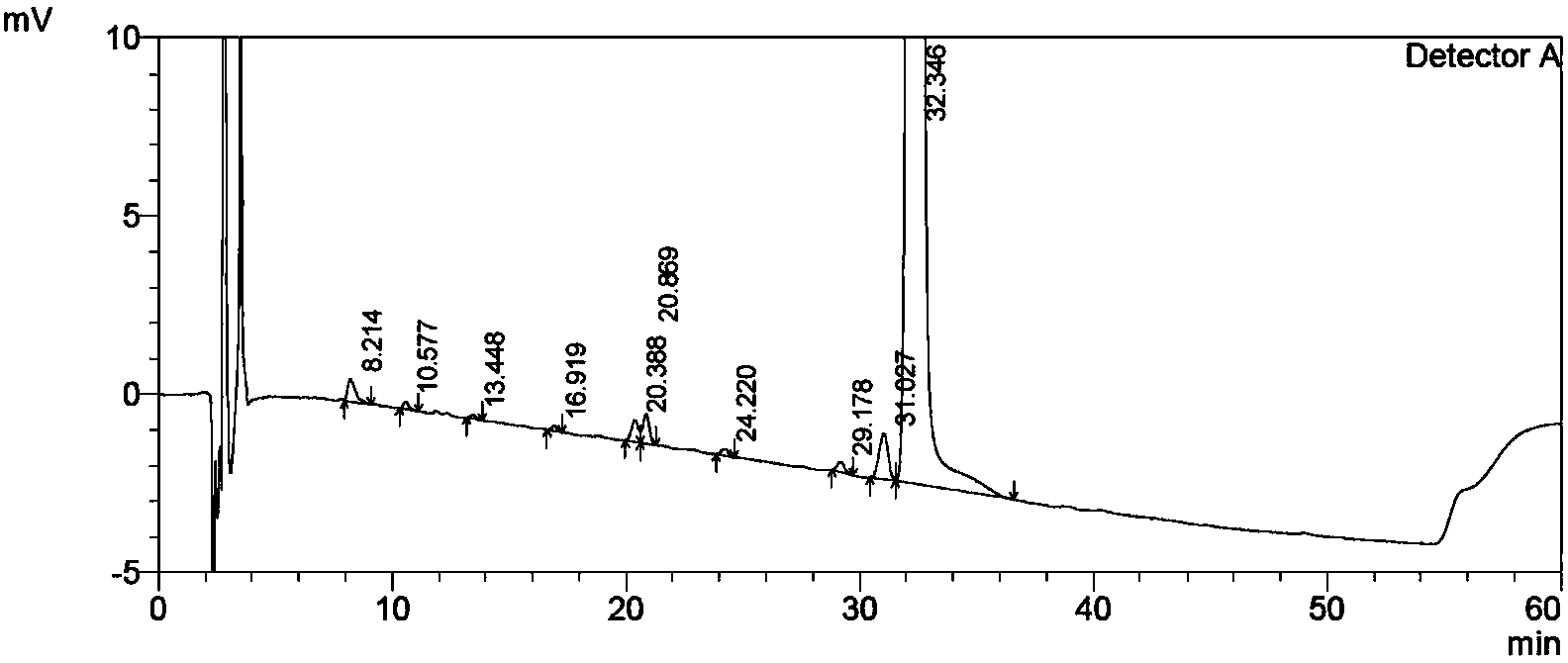
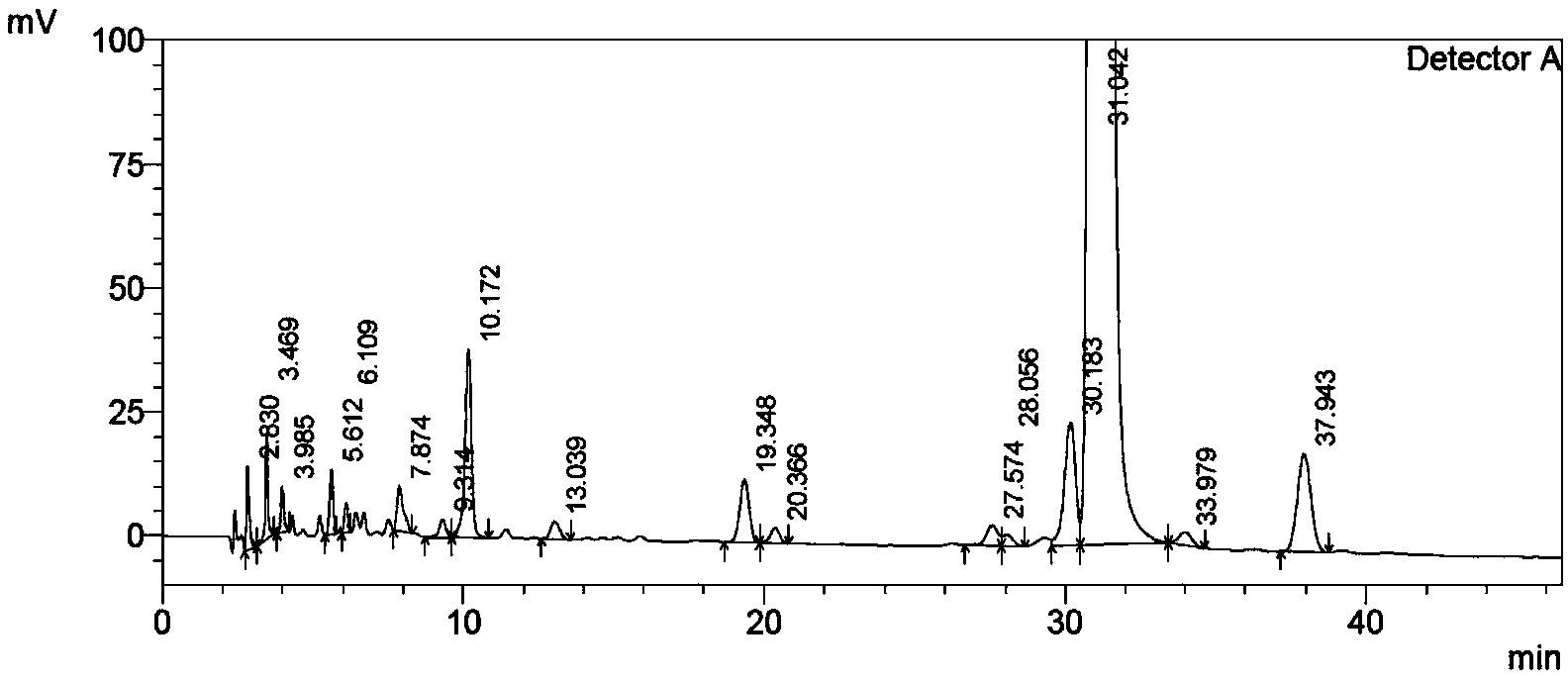
[0056] HPLC content: 98.4%; (see Table 1 and attached figure 1 );
[0057] Mass spectrometry: Instrument model: AccuTOF CS / CADM-YQ-003
[0058] According to theoretical calculation, its [M+]=312.18378, [M+H]=313.19160; low-resolution mass spectrum shows its [M+H]=313.2, [M+Na]=335.2, [M+K]=351.2, which is consistent with The nitrogen oxides have the same ...
Embodiment 2
[0063] The influence of embodiment 2 different reaction temperature conditions on the synthesis of isoquinoline nitrogen oxides
[0064] According to the method in Example 1, explore different reaction temperature conditions (as shown in Table 1) to palonosetron metabolite (3aS, 3'S)-2-[-1-azabicyclo[2.2.2]octyl- The influence of 3-yl]-2,3,3a,4,5,6-hexahydro-1-oxo-1H-benzo[de]isoquinoline nitrogen oxide synthesis (that is, the reaction temperature is different, and the others are the same) , the results are shown in Table 2.
[0065] Table 2 Effects of different reaction temperature conditions on the synthesis of isoquinoline nitrogen oxides
[0066] Reaction temperature (°C)
Embodiment 3
[0067] The influence of embodiment 3 different oxidants on the synthesis of isoquinoline nitrogen oxides
[0068] According to the method in Example 1, explore the effect of different oxidants (as shown in Table 2) on palonosetron metabolite (3aS, 3'S)-2-[-1-azabicyclo[2.2.2]octane-3- base]-2,3,3a,4,5,6-hexahydro-1-oxo-1H-benzo[de]isoquinoline nitrogen oxide synthesis, the results are shown in Table 3.
[0069] Table 3 Effects of different oxidants on the synthesis of isoquinoline nitrogen oxides
[0070] Reaction material
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com