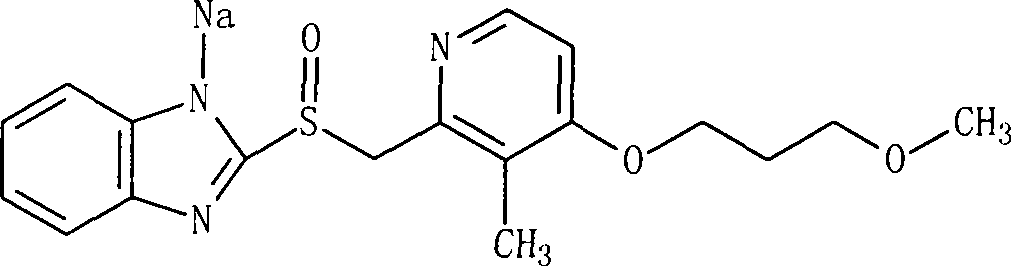
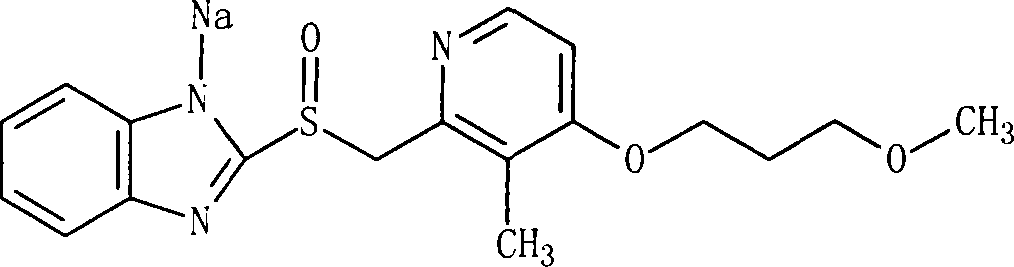
Sodium rebeprazole freeze-dried injection
A technology of rabeprazole sodium and freeze-dried powder injection, which is applied in the direction of freeze-dried transportation, powder transportation, medical preparations of non-active ingredients, etc., to achieve considerable economic and social benefits, uniform and stable quality, and convenient storage and transportation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0022] Example 1:
[0023] prescription
[0024] Rabeprazole Sodium 21.3g
[0025] Meglumine 2g
[0026] Add water for injection to 2000ml
[0027]
[0028] 1,000 bottles total
[0029] Take the prescription amount of meglumine and place it in a sterile container, add water for injection to dissolve and mix uniformly, then add rabeprazole sodium, stir to dissolve and mix uniformly, determine the content of intermediates, and after passing the test in aseptic conditions Bottom, filter with 0.22μm microporous filter membrane until clear, fill the filtrate in a sterile vial, partially plugged with butyl rubber stopper, plate, freeze-dry, stopper, discharge, crimp, quality inspection, Rabeprazole sodium freeze-dried powder injection can be obtained in the package.
Example Embodiment
[0030] Example 2:
[0031] prescription
[0032] Rabeprazole Sodium 21.3g
[0033] Meglumine 4g
[0034] Sodium sulfite 2g
[0035] Add water for injection to 2000ml
[0036]
[0037] 1,000 bottles total
[0038] Take the prescription amount of meglumine and place it in a sterile container, add water for injection to dissolve and mix uniformly, then add rabeprazole sodium and sodium sulfite, stir to dissolve and mix uniformly, determine the intermediate content. Under the condition of bacteria, filter with 0.22μm microporous filter membrane to clear, fill the filtrate in sterile vials, partially plugged with butyl rubber stopper, plate, freeze-dry, stopper, take out the box, crimp, quality After inspection, the package is ready to get rabeprazole sodium freeze-dried powder injection.
Example Embodiment
[0039] Example 3:
[0040] prescription
[0041] Rabeprazole Sodium 21.3g
[0042] Meglumine 6g
[0043] Mannitol 10g
[0044] Sodium bisulfite 2g
[0045] Add water for injection to 2000ml
[0046]
[0047] 1,000 bottles total
[0048] Take the prescription amount of meglumine and place it in a sterile container, add water for injection to dissolve and mix well, then add rabeprazole sodium, mannitol and sodium bisulfite, stir to dissolve and mix well, determine the intermediate Content, after qualified, filter with 0.22μm microporous filter membrane to clear under aseptic conditions, fill the filtrate in sterile vials, partially stopper with butyl rubber stopper, plate, freeze-dry, stopper, and discharge Box, crimping, quality inspection, and packaging to get rabeprazole sodium freeze-dried powder injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com