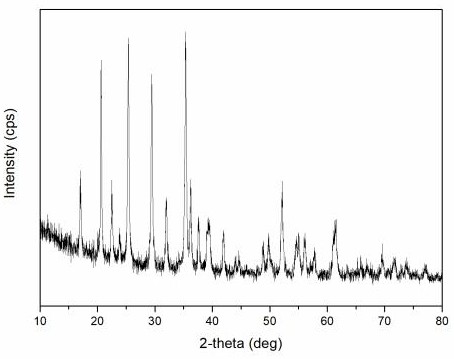
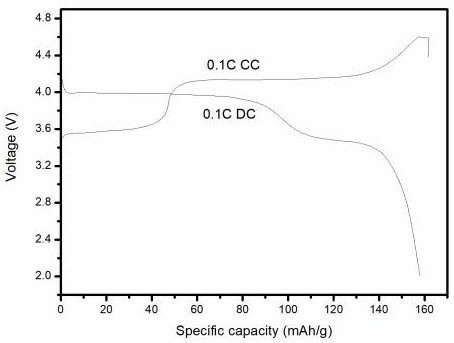
Preparation method of lithium manganese iron phosphate positive electrode material
A technology of lithium iron manganese phosphate and manganese phosphate is applied in the field of positive electrode materials of lithium ion batteries to achieve the effects of good electrical performance, high voltage platform and excellent cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A preparation method for lithium manganese iron phosphate, which comprises the following steps:
[0032] First add 100 g of pure water to the ball mill, weigh 10.8 g of ammonium dihydrogen phosphate, and then slowly add 4.98 g of lithium carbonate, the reaction between ammonium dihydrogen phosphate and lithium carbonate will produce a lot of bubbles, wait until the reaction is complete without bubbles After generation, add 6 g of anhydrous iron phosphate, 4.8 g of polyethylene glycol, 0.08 g of titanium dioxide, 0.1 g of ammonium metavanadate, 6.9 g of trimanganese tetraoxide, and 0.15 g of titanate coupling agent, ball milled for 1 h, and then transferred to sand mill, the final particle size of sand mill D50 is 236 nm, so that the raw materials such as iron source, lithium source, manganese source, phosphorus source, carbon source, metal ion dopant and so on are fully mixed evenly, Then granulate by spray drying to obtain brown precursor powder. The precursor was pac...
Embodiment 2
[0034] A preparation method for lithium manganese iron phosphate, which comprises the following steps:
[0035] First add 100 g of pure water to the ball mill, weigh 12.2 g of diammonium hydrogen phosphate, and then slowly add 4.98 g of lithium carbonate, the reaction between ammonium dihydrogen phosphate and lithium carbonate will produce a lot of bubbles, wait until the reaction is complete without bubbles After production, add 6 g of anhydrous iron phosphate, 4.8 g of polyethylene glycol, 0.1 g of titanium dioxide, 6.9 g of trimanganese tetraoxide, 0.15 g of titanate coupling agent, ball mill for 1 hour, and then transfer to Sand milling, the final particle size of sand milling D50 is 263 nm, fully mix the raw materials such as iron source, lithium source, manganese source, phosphorus source, carbon source, metal ion dopant, etc., and then use spray drying to granulate to obtain brown precursor powder. The precursor was packed in a graphite sagger and sintered at a high te...
Embodiment 3
[0037] A preparation method for lithium manganese iron phosphate, which comprises the following steps:
[0038] First add 100 g of pure water to the ball mill, weigh 7.1 g of diammonium hydrogen phosphate, and then slowly add 3.3 g of lithium carbonate, the reaction between ammonium dihydrogen phosphate and lithium carbonate will produce a lot of bubbles, wait until the reaction is complete without bubbles After production, add 4 g of anhydrous iron phosphate, 0.4 g of polyethylene glycol, 1.4 g of glucose, 0.03 g of titanium dioxide, 16.3 g of manganese acetate tetrahydrate, 0.2 g of titanate coupling agent, and ball mill 1 h, then transfer to sand mill, the final particle size of sand mill D50 is 245nm, fully mix raw materials such as iron source, lithium source, manganese source, phosphorus source, carbon source, metal ion dopant, and then spray dry Granulate to obtain brown precursor powder. The precursor was packed in a graphite sagger and sintered at a high temperature ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com