Cucurbitacin nano preparation comprising protein, preparation method and use thereof
A nano-preparation, cucurbitacin technology, applied in the field of medicine, can solve the problems that have not yet been reported on the research of cucurbitacin protein nanoparticles, and achieve the effects of improving drug efficacy, excellent emulsifying ability, and improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
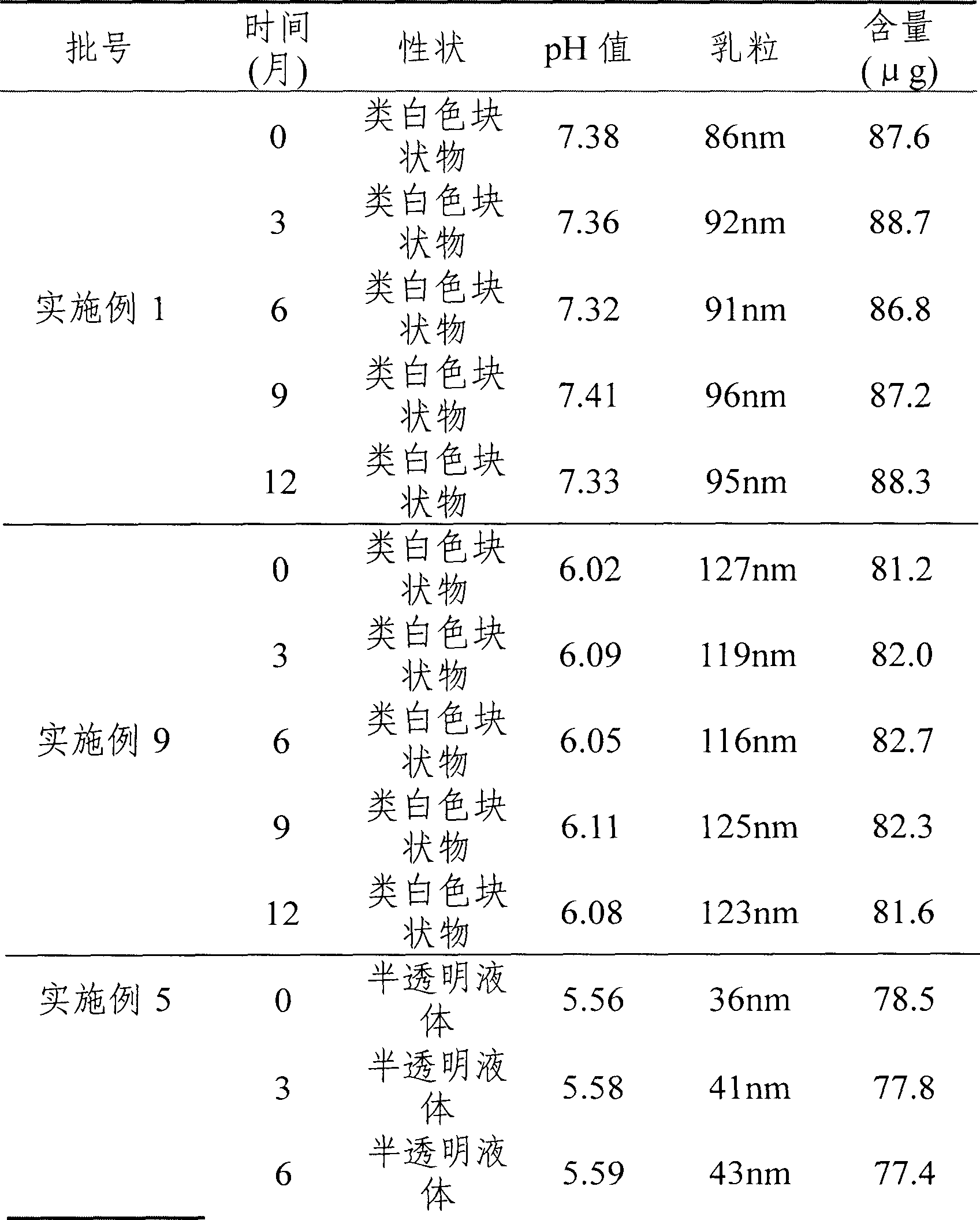
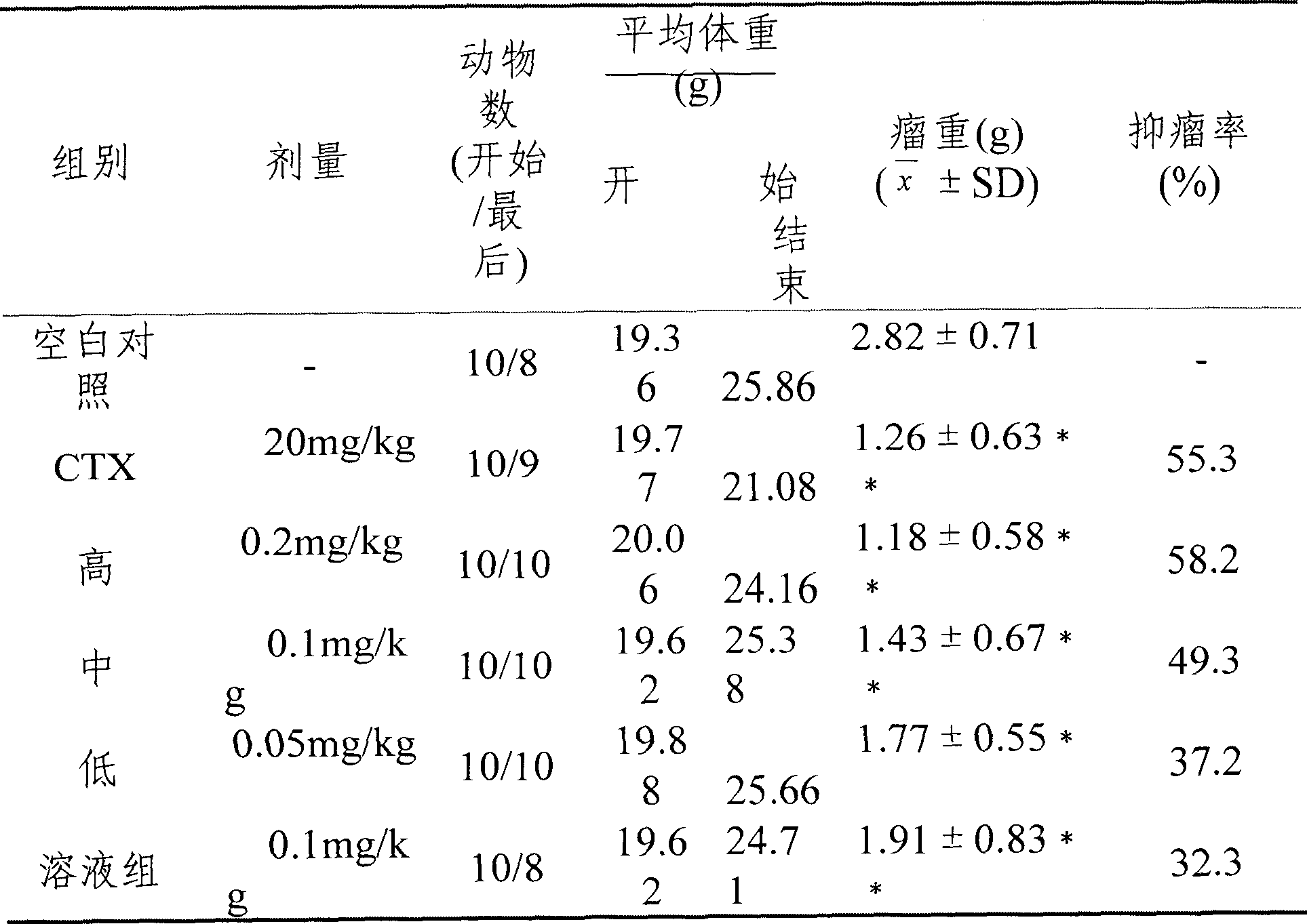
[0020] Example 1: Cucurbitacin BE (effective extraction part of cucurbitacin, cucurbitacin B content greater than 60%) prescription composition: 0.1 g of cucurbitacin, 5 g of human serum albumin, appropriate amount of dichloromethane, and the rest is water for injection, 100 ml in total.
[0021] Preparation Process:
[0022] Weigh the prescribed amount of cucurbitacin, dissolve it with dichloromethane to make a solution, and set aside. Take commercially available human serum albumin (20%) and dilute it with water for injection into a 5% solution, adjust the pH value to 7.5 with acid or alkali, and set aside. Mix cucurbitacin dichloromethane solution and human serum albumin solution at 0°C, emulsify, and prepare colostrum. Then the colostrum is dispersed through a homogenizer (or micro jet), the first step is to adjust the homogenization pressure to 5000psi, and the second step is to adjust to 35000psi, and the average particle size of online detection is 5nm-200nm to obtain ...
Embodiment 2
[0024] Example 2, commercially available cucurbitacin BE (the effective extraction part of cucurbitacin, the content of cucurbitacin B is greater than 60%).
[0025] Prescription composition: 0.01g of cucurbitacin, 1g of human serum albumin, 1g of poloxamer F681, appropriate amount of chloroform, 10g of sucrose, and the rest is water for injection, 100ml in total.
[0026] Preparation Process:
[0027] Weigh the prescribed amount of cucurbitacin, dissolve it with chloroform to make a solution, and set aside. Take 10g of sucrose, dissolve it in water for injection, add 1g each of human serum albumin and poloxamer F68 to make a solution, adjust the pH value to 3 with citric acid, and set aside. Mix cucurbitacin chloroform solution and human serum albumin solution at 10°C, emulsify, and prepare colostrum. Then the colostrum is dispersed through a homogenizer (or micro jet), the first step is to adjust the homogenization pressure to 2000psi, and the second step is to adjust to 3...
Embodiment 3
[0028] Embodiment 3, refined cucurbitacin BE (cucurbitacin B content is greater than 80%)
[0029] Recipe: 0.05g of refined cucurbitacin BE, 0.05g of human serum albumin, 1g of poloxamer, appropriate amount of ethyl acetate, 5g of mannitol, 10g of trehalose, and the rest is water for injection, 100ml in total.
[0030] Preparation process: Weigh the refined cucurbitacin of the prescribed amount, dissolve it with ethyl acetate to make a solution, and set aside. Weigh 0.05 g of human serum albumin and 1 g of poloxamer, dissolve it with water for injection to make a solution, add the prescribed amount of mannitol and trehalose, adjust the pH value to 9 with 0.01M sodium hydroxide after dissolving, and set aside. Mix and emulsify cucurbitacin ethyl acetate solution and human serum albumin solution at 0°C to prepare colostrum. Then the colostrum is dispersed through a homogenizer (or micro-jet), the first step is to adjust the homogenization pressure to 5000psi, and the second ste...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com