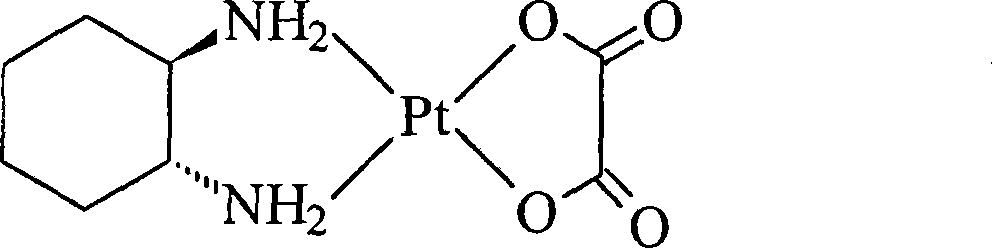
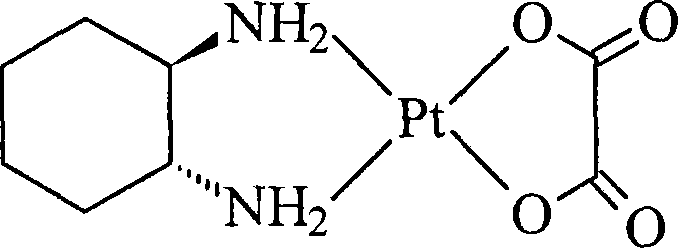
Oxaliplatin lyophilized powder injection and preparing method thereof
A technology of freeze-dried powder injection and oxaliplatin, which is applied in the field of oxaliplatin freeze-dried powder injection, can solve the problems of increased risk of cross-contamination, injury of operators, and failure to propose improvements, etc., to reduce the risk of cross-contamination , avoid damage, and reduce the bottle explosion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Ordinary method to prepare oxaliplatin freeze-dried powder injection (50mg specification)
[0023] prescription
[0024] Oxaliplatin 50g
[0025] Mannitol 300g
[0026] Add water for injection to 10000ml
[0027]
[0028] A total of 1000 bottles were made
[0029] Put oxaliplatin in a container, add 80% water for injection, stir to dissolve and mix evenly, then add mannitol, stir to dissolve and mix evenly, measure the content of intermediates, and use water for injection to make up the volume after passing the test To the full amount, under sterile conditions, filter with a 0.22 μm microporous membrane until clear, fill the filtrate into a sterile 25ml vial, partially plug it with a butyl rubber stopper, put it into a plate, send it to a freeze dryer, and close it. Open the chamber door, open the freeze dryer, and use heat transfer oil to cool the plate layer to lower the product temperature. When the sample reaches -40°C, stop the ...
Embodiment 2
[0031] Ordinary method to prepare oxaliplatin freeze-dried powder injection (100mg specification)
[0032] prescription
[0033] Oxaliplatin 100g
[0034] Mannitol 600g
[0035] Add water for injection to 20000ml
[0036]
[0037] A total of 1000 bottles were made
[0038] Put oxaliplatin in a container, add 80% water for injection, stir to dissolve and mix evenly, then add mannitol, stir to dissolve and mix evenly, measure the content of intermediates, and use water for injection to make up the volume after passing the test To the full amount, under sterile conditions, filter with a 0.22 μm microporous membrane until clear, fill the filtrate into a sterile 30ml vial, partially plug it with a butyl rubber stopper, put it into a plate, send it to a freeze dryer, and close it. Open the chamber door, open the freeze dryer, and use heat transfer oil to cool the plate layer to lower the product temperature. When the sample reaches -40°C, stop th...
Embodiment 3
[0040] prescription
[0041] Oxaliplatin 50g
[0042]Mannitol 300g
[0043] Citric acid 20g
[0044] Sodium citrate 41.7g
[0045] Add water for injection to 10000ml
[0046]
[0047] A total of 1000 bottles were made
[0048] Take oxaliplatin in a container, add 80% water for injection, stir to dissolve and mix evenly, then add mannitol, citric acid and sodium citrate, stir to dissolve and mix evenly, and determine the intermediate After passing the test, dilute to the full volume with water for injection. Under sterile conditions, filter it with a 0.22 μm microporous membrane until it becomes clear. Fill the filtrate into a sterile 25ml vial, and partially plug it with a butyl rubber stopper. The plate is sent to the freeze dryer, the door is closed, the freeze dryer is turned on, and the heat transfer oil is used to cool the plate layer to lower the product temperature. When the sample reaches -40°C, stop the plate cooling and turn on t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com