1,3-benzothiazinone compound as well as synthesis method and application thereof
A technology of benzothiazinone and synthesis method, which is applied in the field of medicinal chemistry, can solve the problems of toxicity and interleukin compensatory increase, and achieve the effect of low toxicity, low toxicity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
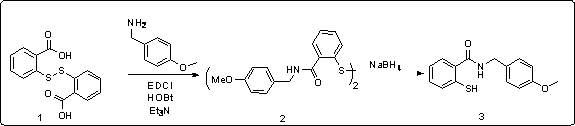
[0035] N-(4-methoxybenzyl)-2-(methylthio)benzamide (Formula 2):
[0036] 1 2 g (6.5 mmol) of 2,2'-dithiodibenzoic acid, 3 g of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDCl, 15.7 mmol), 2 g of 1-hydroxybenzotriazole (HOBt, 15.7 mmol) and 2.7 g (19.7 mmol) of 4-methoxybenzyl were placed in a 250 ml round bottom flask and replaced with argon. After adding 60ml of dry dichloromethane as a solvent, put it in an ice bath and stir, and add 3.3g (32.7mmol) of triethylamine dropwise, and then move to room temperature to react overnight. The reaction solution was quenched with saturated ammonium chloride solution, extracted three times with 50ml of dichloromethane, the solvent was evaporated by rotary evaporation, and the product 2 was separated by column chromatography to obtain 1.1g with a yield of 30%.
Embodiment 2
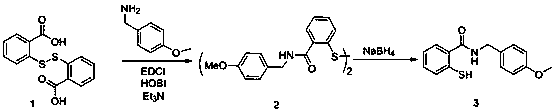
[0038] 2-mercapto-N-(4-methoxybenzyl)benzamide (Formula 3):
[0039]Put compound 2 2,2'-dithio-(4-methoxybenzyl)benzamide 1.1g (2.0mmol) in a 100ml round bottom flask, add 20ml of dry tetrahydrofuran under the protection of argon, and stir in an ice bath . Then slowly add 300 mg (8.0 mmol) of sodium borohydride, and quench the reaction with 5M / L hydrochloric acid under ice-cooling after 5 minutes. After the tetrahydrofuran was evaporated by rotary evaporation, it was extracted three times with dichloromethane, the solvent was evaporated by rotary evaporation, and 1 g of the product was obtained by silica gel column chromatography with a yield of 90%.
Embodiment 3
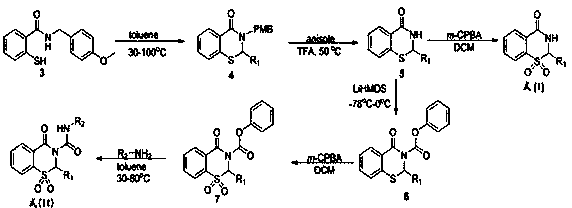
[0041] 3-(4-methoxybenzyl)-2-methyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (formula 4, R 1 =CH 3 ):
[0042] Add compound 3 2-mercapto-(4-benzylmethoxybenzyl) benzamide 1g (3.6mmol), p-toluenesulfonic acid 620mg (3.6mmol), 160mg acetaldehyde (3.6mmol) and 35ml toluene in a 100ml round bottom flask , react overnight at 30°C under the protection of argon. After TLC traced the disappearance of the raw material, the toluene was evaporated by rotary evaporation, and extracted three times with dichloromethane. After the solvent was evaporated, the column was separated by column chromatography to obtain 750 mg of white solid with a yield of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com