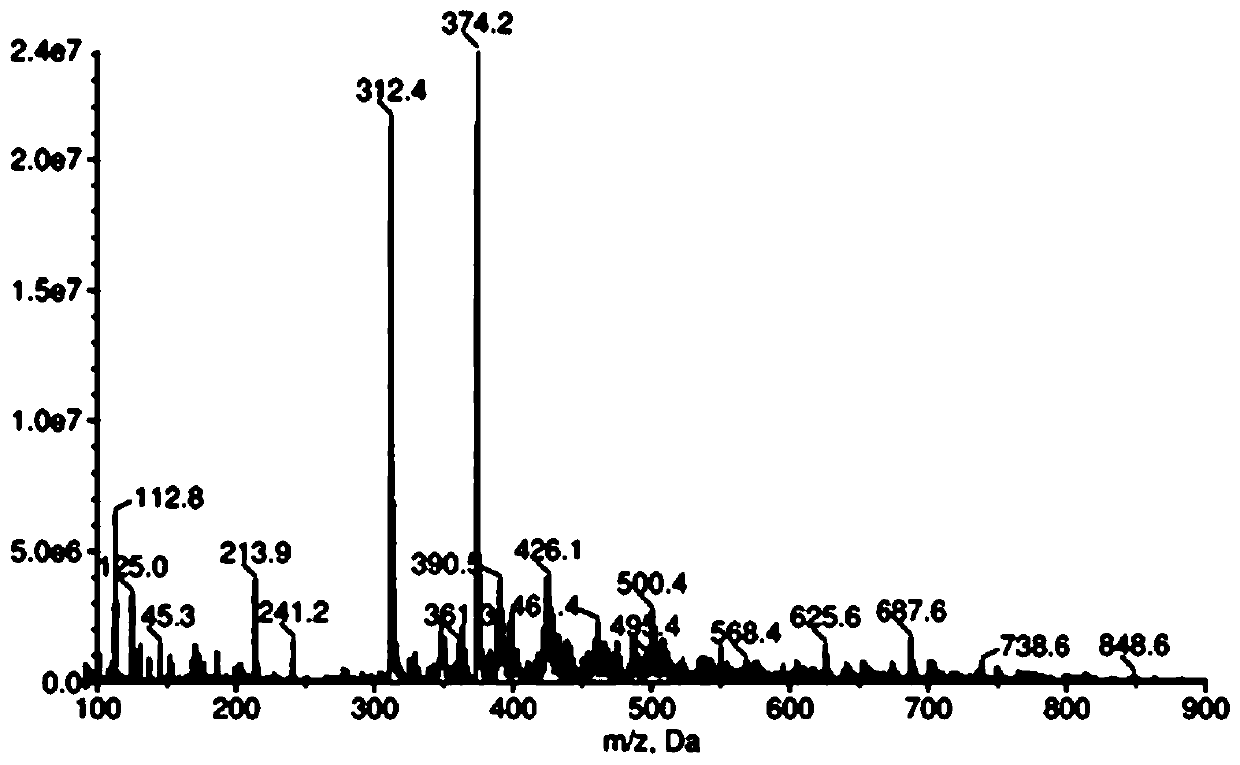
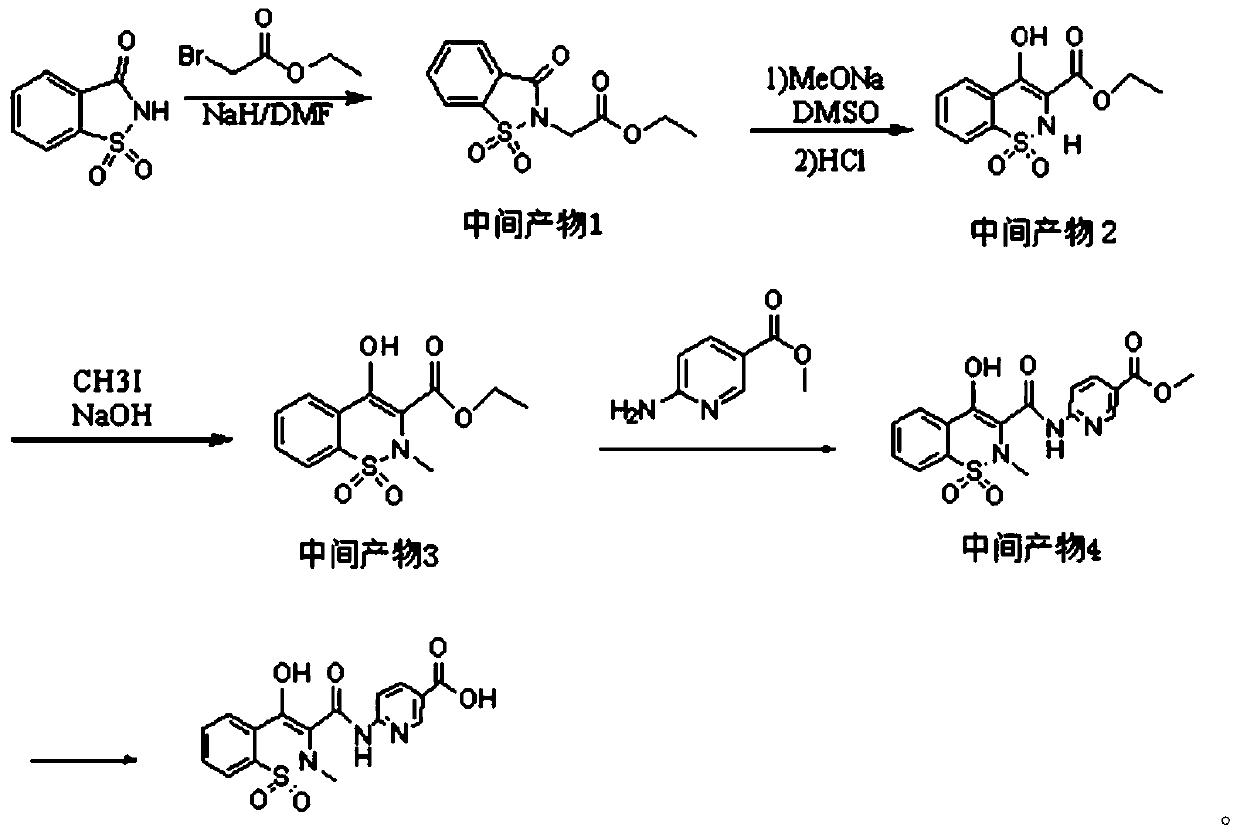
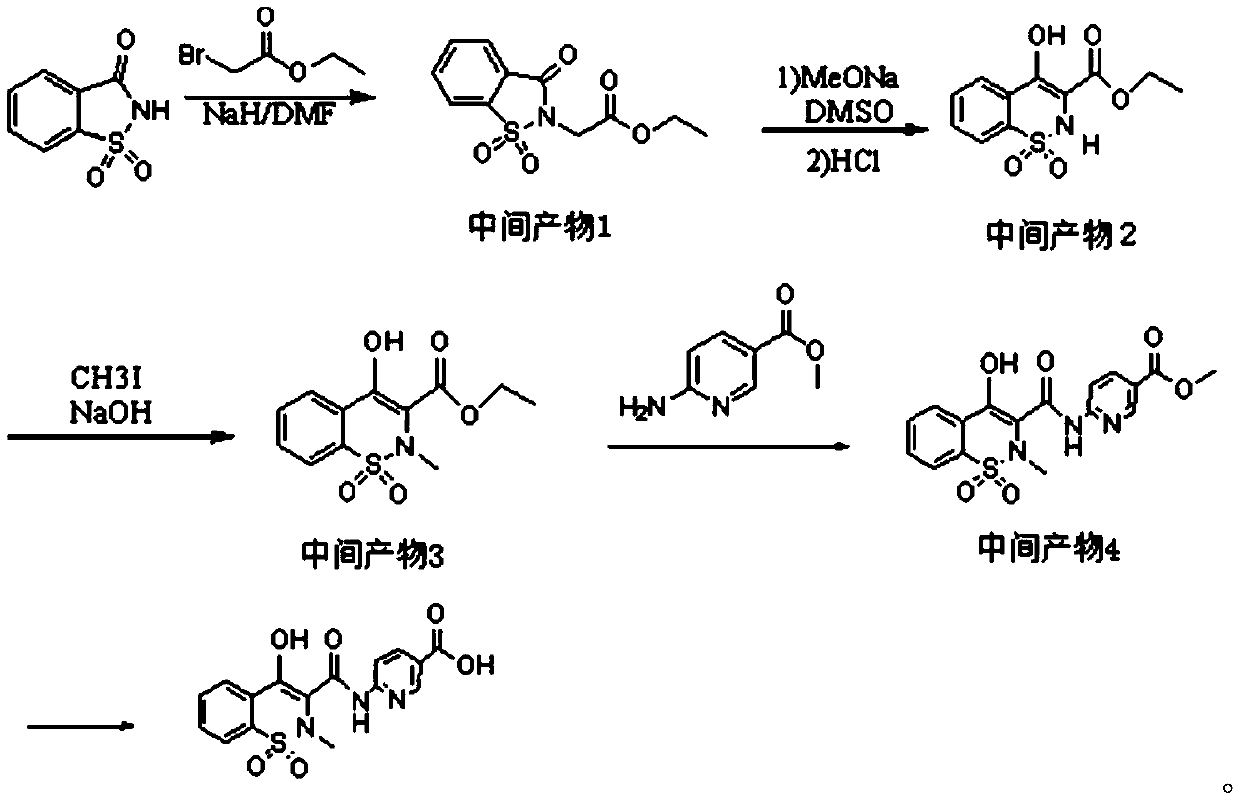
Piroxicam hapten and preparation method and application thereof
A technology of piroxicam and hapten, which is applied in the direction of material inspection products, instruments, analytical materials, etc., to achieve the effect of simple raw material components, low detection cost, and easy experimental operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A kind of preparation method of piroxicam hapten:
[0034] 1) Take saccharin and ethyl bromoacetate mixed reaction to make intermediate product 1:
[0035] Weigh 13g of saccharin and dissolve it in 25mL of DMF, add 1.8g of NaH in batches under an ice bath, stir for 10min, then slowly add 6.5g of ethyl bromoacetate dropwise, react at room temperature for 1h, and then react at 80°C for 2.5h. After cooling down in an ice bath, pour the mixture into 100ml of ice water, filter the precipitated solid with suction, wash with water, and dry to obtain a white solid powder which is the intermediate product 1;
[0036] 2) intermediate product 2 is obtained after mixing intermediate product 1 with DMSO and MeONa and extracting:
[0037] Add 6g of intermediate product 1 to a mixed solution of DMSO (23ml) and MeONa (2.60g), control the temperature at less than 30°C, stir for 1h, and the color of the solution changes from yellow to brown; pour the reaction solution into 50ml of 3N hy...
Embodiment 2
[0045] A kind of preparation method of piroxicam hapten:
[0046]1) Take saccharin and ethyl bromoacetate mixed reaction to make intermediate product 1:
[0047] Weigh 10 g of saccharin and dissolve it in 20 mL of DMF, add 1.5 g of NaH in batches under an ice bath, and slowly add 6.07 g of ethyl bromoacetate dropwise after stirring for 10 min, react at room temperature for 1 h, and then react at 80°C for 2.5 h. After cooling down in an ice bath, pour the mixture into 100ml of ice water, filter the precipitated solid with suction, wash with water, and dry to obtain a white solid powder which is the intermediate product 1;
[0048] 2) intermediate product 2 is obtained after mixing intermediate product 1 with DMSO and MeONa and extracting:
[0049] Add 5g of intermediate product 1 to a mixed solution of DMSO (20ml) and MeONa (2.45g), control the temperature at less than 30°C, stir and react for 1h, the color of the solution changes from yellow to brown; pour the reaction soluti...
Embodiment 3
[0057] A kind of preparation method of piroxicam hapten:
[0058] 1) Take saccharin and ethyl bromoacetate mixed reaction to make intermediate product 1:
[0059] Weigh 20g of saccharin and dissolve it in 40mL of DMF, add 3g of NaH in batches under an ice bath, stir for 10min, then slowly add 12g of ethyl bromoacetate dropwise, react at room temperature for 1h, and then react at 80°C for 2.5h. After cooling down in an ice bath, pour the mixed solution into 200ml of ice water, filter the precipitated solid with suction, wash with water, and dry to obtain a white solid powder which is the intermediate product 1;
[0060] 2) intermediate product 2 is obtained after mixing intermediate product 1 with DMSO and MeONa and extracting:
[0061] Add 10g of intermediate product 1 to a mixed solution of DMSO (40ml) and MeONa (5g), control the temperature at less than 30°C, stir and react for 1h, the color of the solution changes from yellow to brown; pour the reaction solution into 100ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com