Imidacloprid hapten, complete antigen and synthesis and application of imidacloprid hapten and complete antigen
A complete antigen, imidacloprid technology, applied in the direction of animal/human protein, serum albumin, ovalbumin, etc., can solve the problems of endangering the environment or human health, entering the soil or human body, complex reaction conditions, etc., to save synthesis costs, The synthesis method is simple and efficient, and the effect of efficient and accurate detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] The synthetic method of this imidacloprid hapten comprises the steps:
[0051] S1. Preparation of imidacloprid intermediate
[0052] Dissolving imidacloprid in DMF, adding sodium hydride and tert-butyl halocarboxylate, obtains the tert-butyl carboxylate intermediate of imidacloprid N substitution, wherein, the molar ratio of imidacloprid, sodium hydride and tert-butyl halocarboxylate is 1:1-1.1:1-3;
[0053] S2. Preparation of imidacloprid hapten
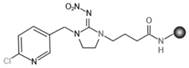
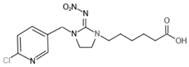
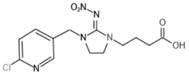
[0054] Under acidic conditions and deprotection reagent environment, imidacloprid N-substituted tert-butyl carboxylate intermediate undergoes de-tert-butyl reaction to obtain imidacloprid hapten, and the deprotection reagent is hydrochloric acid, trifluorohydrochloric acid, hydrobromic acid One, the imidacloprid hapten is imidacloprid hapten derivative 2-[3-[(6-chloropyridyl-3-yl)methyl]-2-nitroimidazolin-1-yl]acetic acid, 3-[3-[(6-chloropyridyl-3-yl)methyl]-2-nitroimidazolin-1-yl]propanoic acid, 4-[3-[(6-chloropyridyl- 3...
Embodiment 1
[0071] The imidacloprid hapten synthesized in this example is 2-[3-[(6-chloropyridyl-3-yl)methyl]-2-nitroimidazolin-1-yl]acetic acid (hapten 1).
[0072] This embodiment relates to a kind of imidacloprid hapten and its synthesis method:
[0073] S1. Preparation of imidacloprid intermediate
[0074] Weigh 5g of imidacloprid and dissolve it in 20ml of DMF, stir, add 0.469g of sodium hydride (60% in mineral oil) in batches, stir at room temperature for 30min, add 7.6344g of tert-butyl bromoacetate dropwise, and stir the reaction Overnight, the reaction solution was slowly poured into 150 ml of ice-saturated saturated ammonium chloride solution, extracted three times with 150 ml of tertiary methyl ether, the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated in vacuo to obtain a crude intermediate;
[0075] S2. Preparation of imidacloprid hapten
[0076] Weigh 0.2g of the intermediate obtained in step S1, slowly add 5...
Embodiment 2
[0080] The imidacloprid hapten synthesized in this example is 3-[3-[(6-chloropyridyl-3-yl)methyl]-2-nitroimidazolin-1-yl]propionic acid (hapten 2) .
[0081] This embodiment relates to a kind of imidacloprid hapten and its synthesis method:
[0082] S1. Preparation of imidacloprid intermediate
[0083] Weigh 5g of imidacloprid and dissolve it in 20ml of DMF, stir well, add 0.469g of sodium hydride (60% in mineral oil) in batches, stir at room temperature for 30min, drop in 8.183g of tert-butyl bromopropionate, and react Stir overnight, slowly pour the reaction solution into 150ml of ice-saturated saturated ammonium chloride solution, extract 3 times with 150ml of tertiary methyl ether, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, and concentrate in vacuo to obtain the crude product of the target product the intermediate;
[0084] S2. Preparation of imidacloprid hapten
[0085] Weigh 0.2g of the intermediate obtained in step S1, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com