Clarithromycin derivatives and their preparation methods and applications
A technology for clarithromycin and derivatives, applied in the field of clarithromycin derivatives, can solve the problems of complicated operation steps, high cost, unsuitable for screening and detection of large-scale samples, etc., and achieves the analysis of large sample volume, low cost and high sensitivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1) Add 750.6mg (1mmol) of clarithromycin into a 50ml reaction bottle, add 10ml of dilute hydrochloric acid, stir at room temperature for 2 hours, add 20ml of water, adjust the pH to 8.0-9.0 with concentrated ammonia water, precipitate a white solid, add solid Saturate the solution with sodium chloride, stir slowly for 30 minutes, filter with suction, take the filter cake and dissolve it in 50ml ethyl acetate, wash 3 times with saturated aqueous sodium chloride solution, take the ethyl acetate layer, and dry it with anhydrous sodium sulfate for 2 hours , filtered, and ethyl acetate was rotary evaporated under reduced pressure to obtain a white solid, which was 432.7 mg of clarithromycin derivative intermediate.
[0040] 2) Add 300.6mg (0.51mmol) of the intermediate prepared in step 1) into a 10ml reaction flask, add 5ml methanol, add 78.8mg (1.5N) of γ-aminobutyric acid, stir magnetically at 60°C for 12 hours, cool, reduce The solvent was evaporated under pressure and 10...
Embodiment 2
[0042] Embodiment 2, preparation clarithromycin artificial antigen
[0043] 1. Synthesis of Clarithromycin Coating Progen
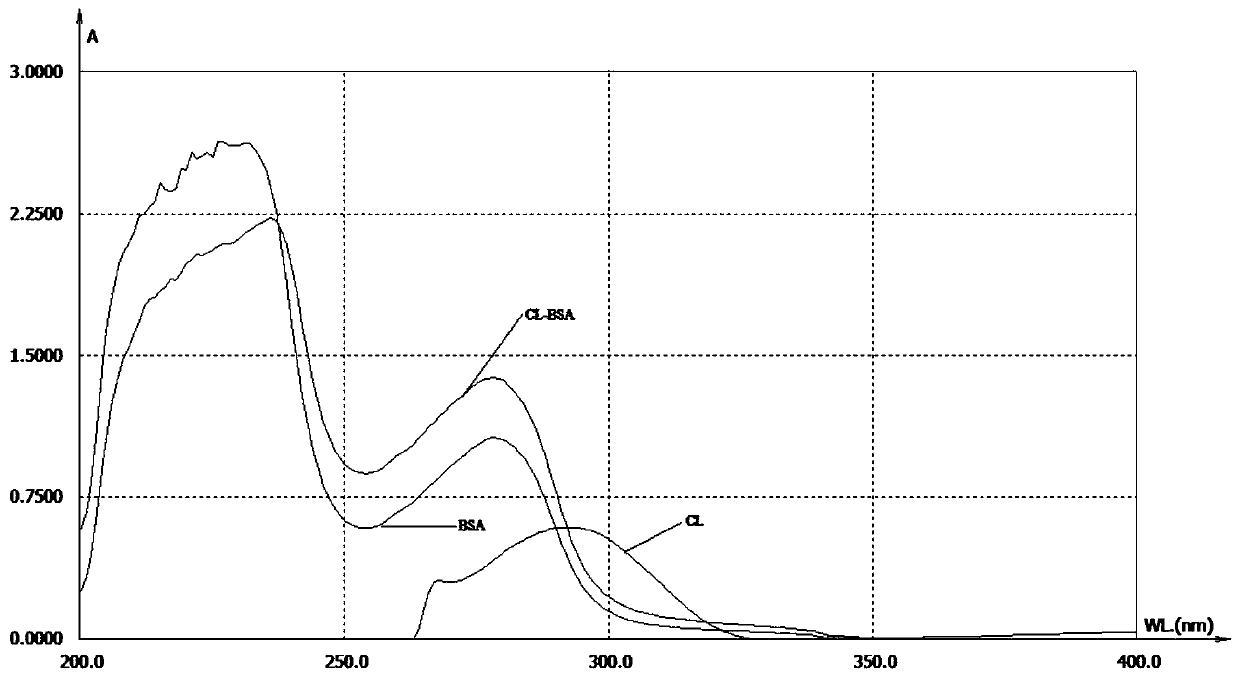
[0044] (1) Dissolve 27.7 mg of the compound shown in formula (I) prepared in Example 1 in 2 ml of N,N-dimethylformamide, add 10 mg of N-hydroxysuccinimide and 10 mg of 1-ethyl-(3- Dimethylaminopropyl) carbodiimide hydrochloride was magnetically stirred at room temperature for 2 h to obtain solution a.
[0045] (2) Add 66 mg of bovine serum albumin into 8 ml of deionized water, stir magnetically at room temperature, and fully dissolve to obtain solution b.
[0046] (3) Add solution a dropwise to solution b, stir slowly at room temperature for 8 hours, then put it into a dialysis bag, dialyze in PBS at 4°C for 72 hours (change the water 5 times in the middle), then centrifuge at 4000rmp for 10 minutes at 4°C, Take the supernatant, i.e. the original clarithromycin-coated solution, divide it into ampoules, and store at -20°C. The original solution is refer...
Embodiment 5
[0063] Example 5, Preparation of clarithromycin polyclonal antibody
[0064] 1. Animal immunity
[0065] New Zealand white rabbits: purchased from the Experimental Animal Center of Guangdong Province.
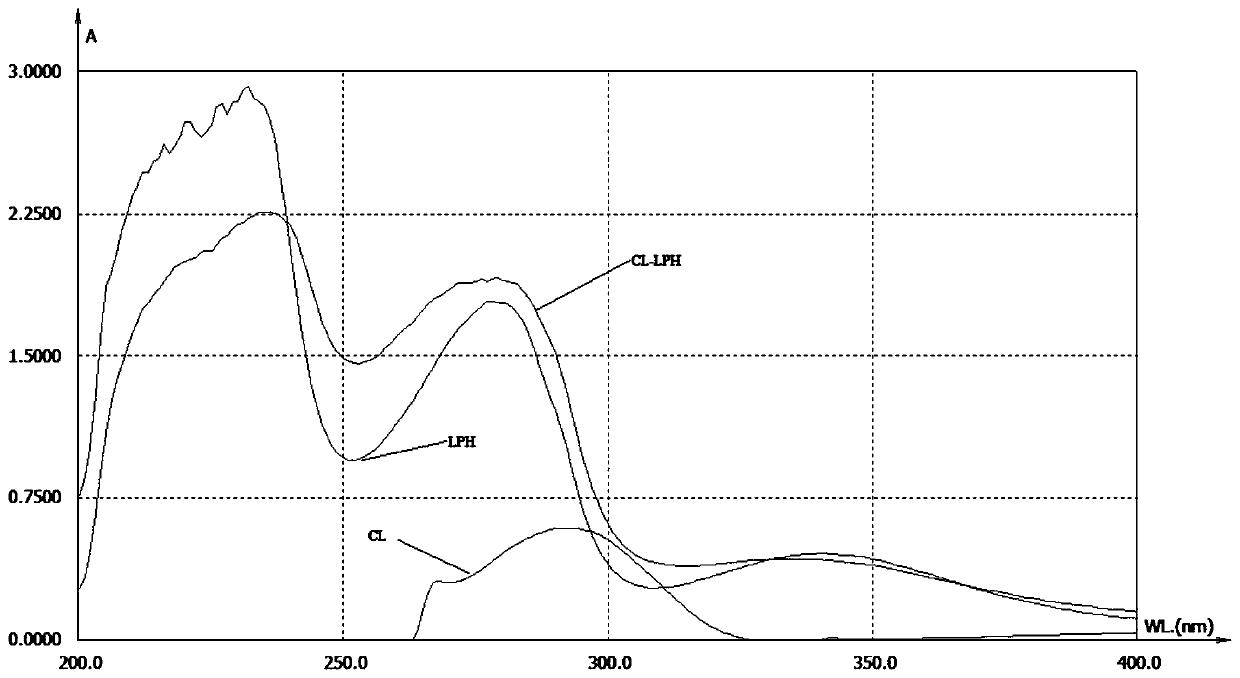
[0066] The New Zealand white rabbits were immunized with the CL-LPH solution prepared in Example 2. The immunization method was multi-point subcutaneous immunization on the back of the spine on both sides. The immunization interval was two weeks, and the dose of each immunization was 1 mg / only, starting from the second immunization On the seventh day after each immunization, blood was collected from the ear vein to detect the serum titer and inhibition. There were eight immunizations in total. After eight immunizations, whole blood was collected from the heart, and the serum was collected by centrifugation to obtain polyclonal antibodies. The collected serum was stored at -20°C.
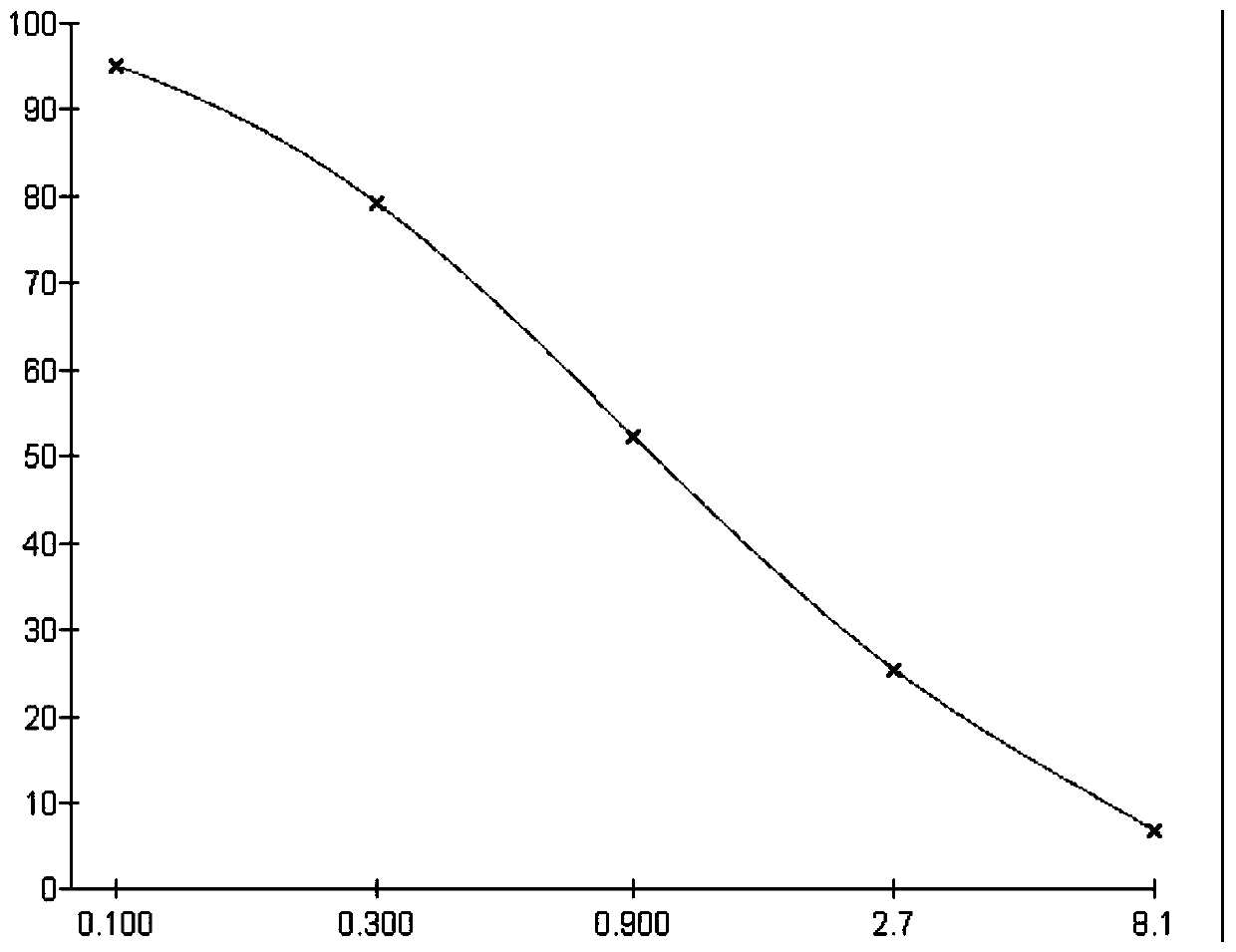
[0067] 2. Identification of polyclonal antibodies
[0068] The polyclonal antibodies obtained i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com