Novel combination and use
a technology of combination and use, applied in the direction of antibacterial agents, antinoxious agents, drug compositions, etc., can solve the problems of no new class of antibiotics marketed for over 37 years, low survival rate of patients suffering acute microbial infections (e.g. tuberculosis or pneumonia), and no new class of antibiotics marketed for over 30 years
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
ard Method
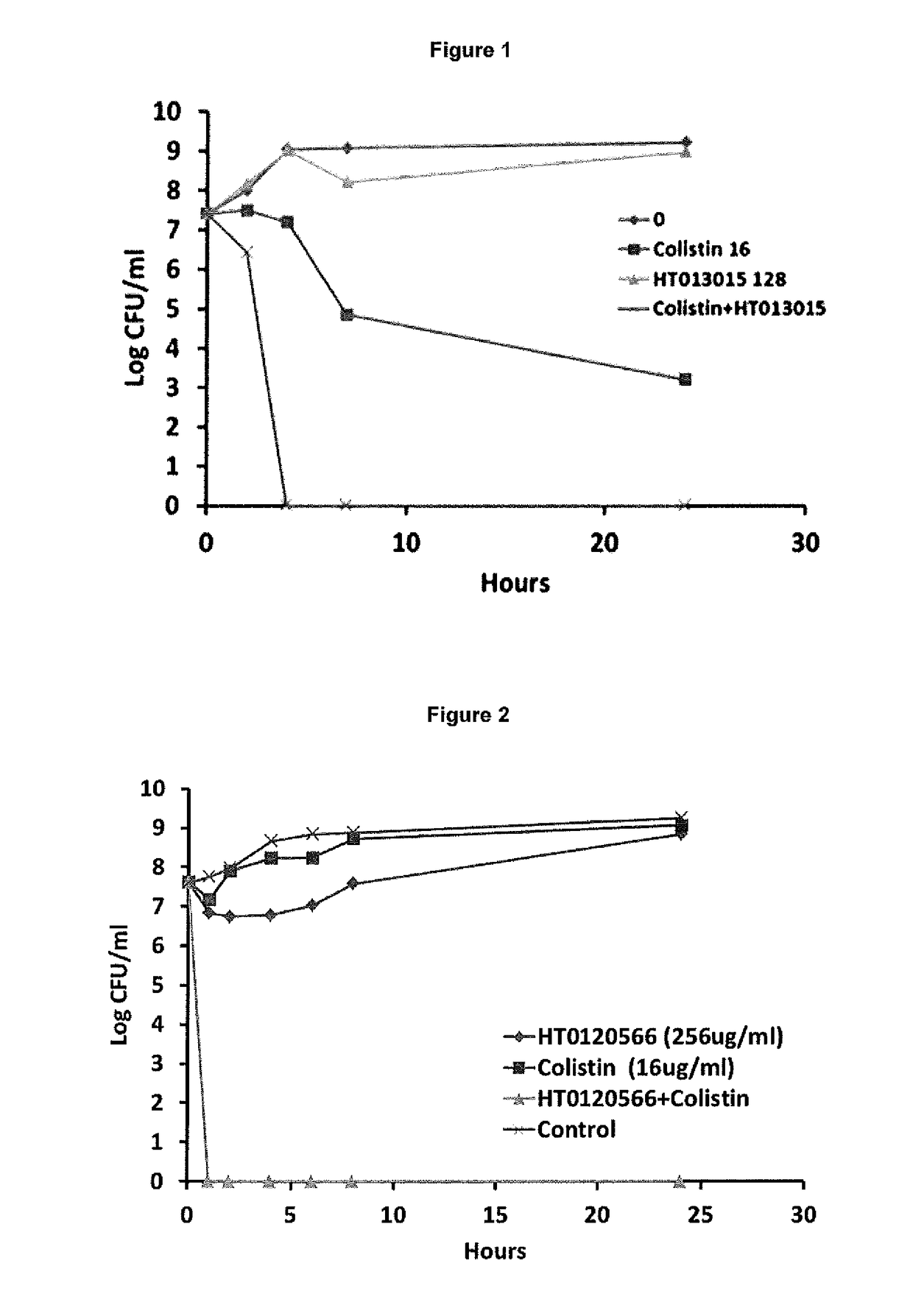
[0159](a) In Vitro Synergy Effect of Colistin and Each of HT013015 (Thymol), HT0121219 (Aspirin), HT0120448 (Ibuprofen), HT0120451 (Indomethacin), HT0120566 (Trifluoperazine Hydrochloride), and HT0121567 (Dichlorophen) Against Log Phase NDM-1 Klebsiella pneumoniae Using the Chequerboard Method
[0160]Growth of Bacteria
[0161]Log phase growth of NDM-1 Klebsiella pneumonia was carried out as described in the art.
[0162]The effects of each combination of the present invention were examined by calculating the fractional inhibitory concentration index (FICI) of each combination, as follows: (MIC of drug A, tested in combination) / (MIC of drug A, tested alone)+(MIC of drug B, tested in combination) / (MIC of drug B, tested alone). The interaction of the combination was defined as showing synergy if the FICI was ≦0.5, no interaction if the FICI was >0.5 but 4.0.
HT01301525612864321684210.50.250Colistin160.400.410.420.420.700.730.740.910.910.410.900.4180.410.420.420.450.451.000.941.071.05...
example 3
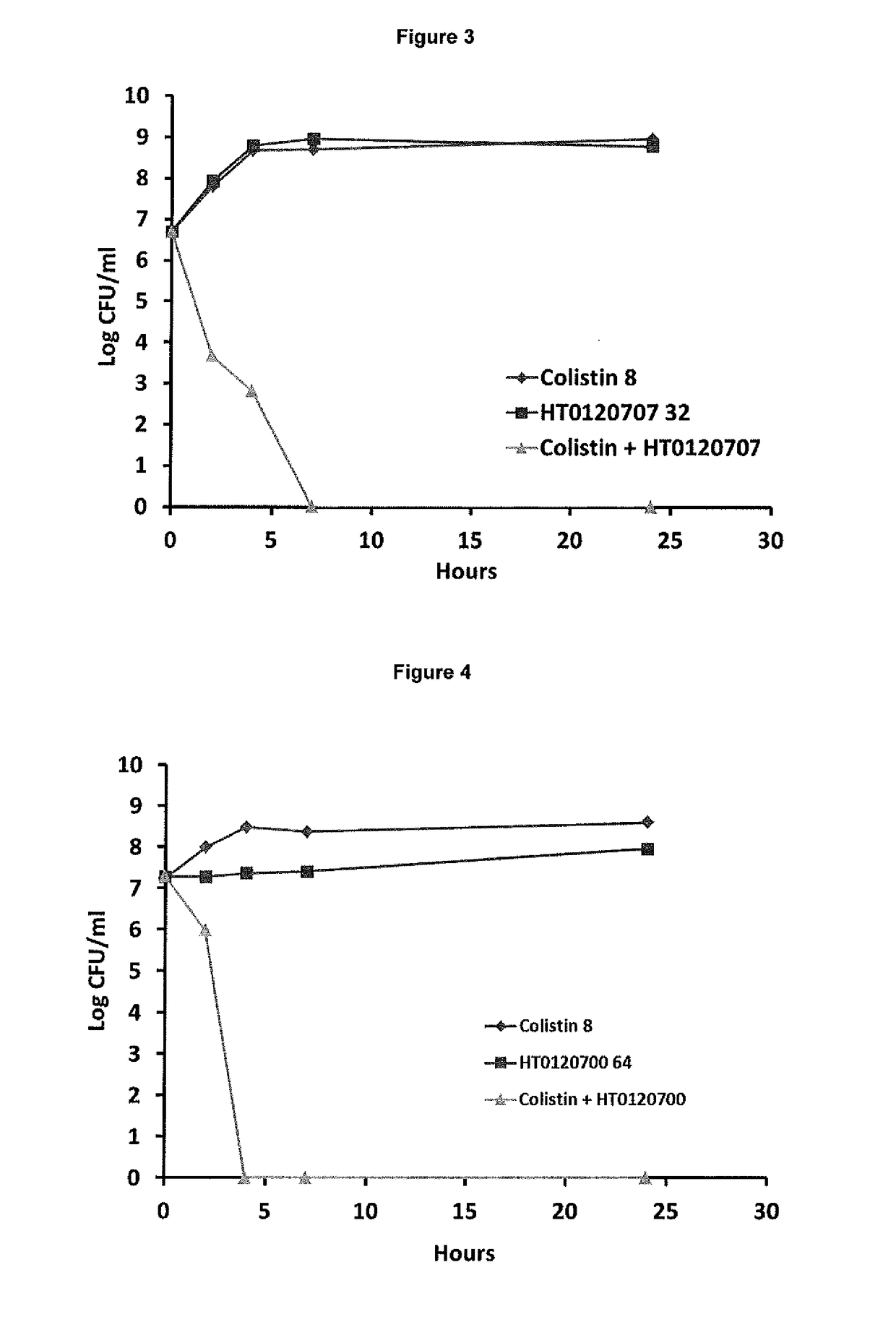
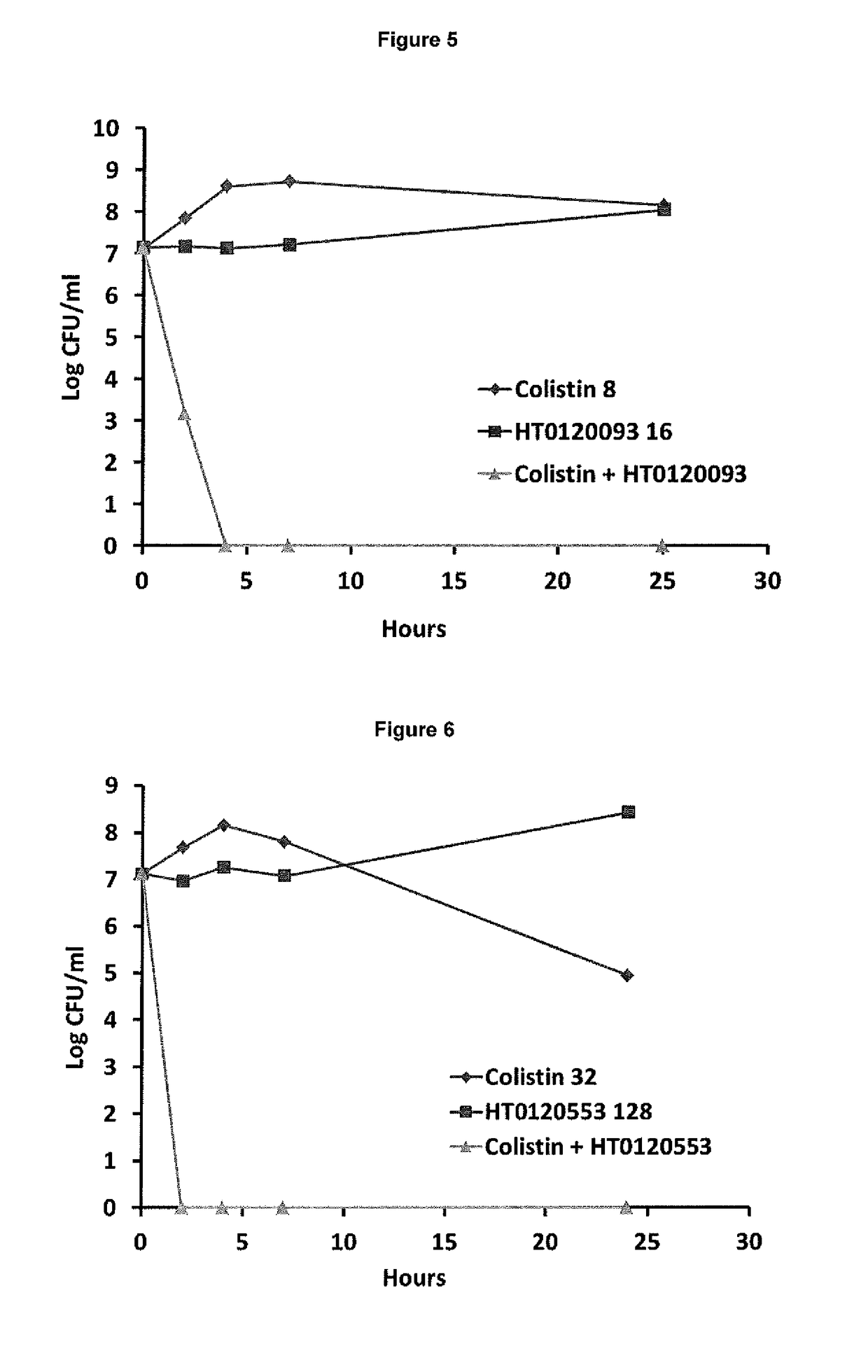
[0168]In Vitro Synergy Effect of Suloctidil in Combination with Colistin Against Log Phase NDM-1 Klebsiella pneumoniae
[0169]The synergistic effect of suloctidil in combination with colistin was tested against log phase NDM-1 Klebsiella pneumoniae using time-kill methods over a period of 24 hours.
[0170]Materials and Methods[0171]Bacterial strain used: NCTC 13443 strain of NDM-1 Klebsiella pneumoniae [0172]Growth of bacteria: Log phase growth of the bacteria was carried out according to methods known in the art.
[0173]Compounds and Preparation:
(i) Suloctidil was obtained from a commercial source and dissolved in DMSO to make a stock concentration of 10 mg / ml.
(ii) Colistin was obtained from a commercial source at a concentration of 20 mg / ml.
[0174]Both suloctidil and colistin were then added to 96 well plates either alone or in the combinations shown below in Table 1.
TABLE 1Agent (Concentration)Number / LetterCombinationCombinationColistin (32 μg / ml)11&A1&CColistin (16 μg / ml)22&A2&CColist...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time- | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com