Piroxicam beta-cyclodextrin inclusion compounds and preparation method of tablets thereof
A technology of cyclodextrin inclusion complex and piroxicam, which is applied in the directions of non-active ingredient medical preparations, active ingredients-containing medical preparations, anti-inflammatory agents, etc., can solve the problems of high production cost, poor flow performance and great difficulty and other problems, to achieve the effects of easy industrialized large-scale production, good liquidity, and simple production and operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
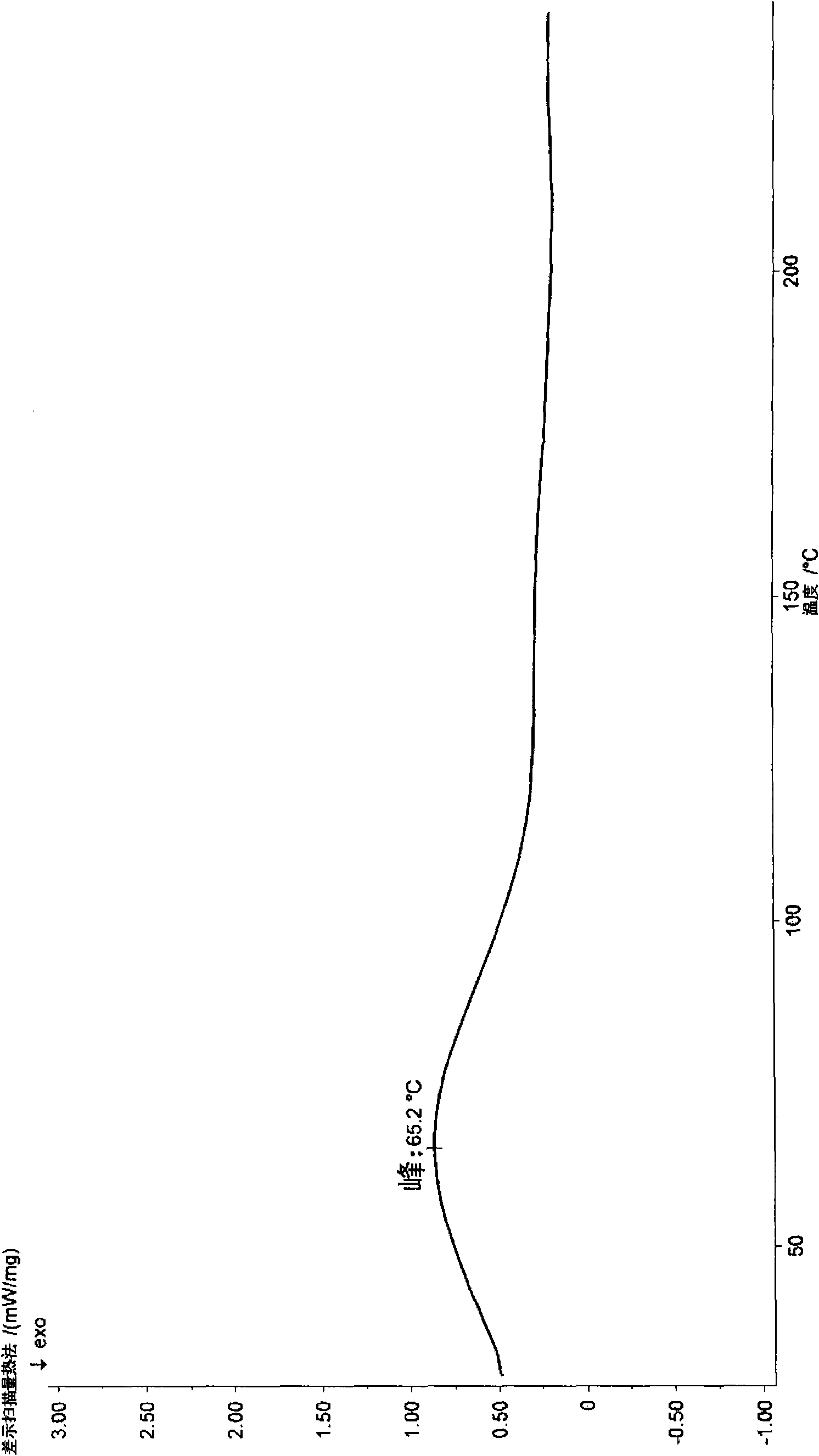
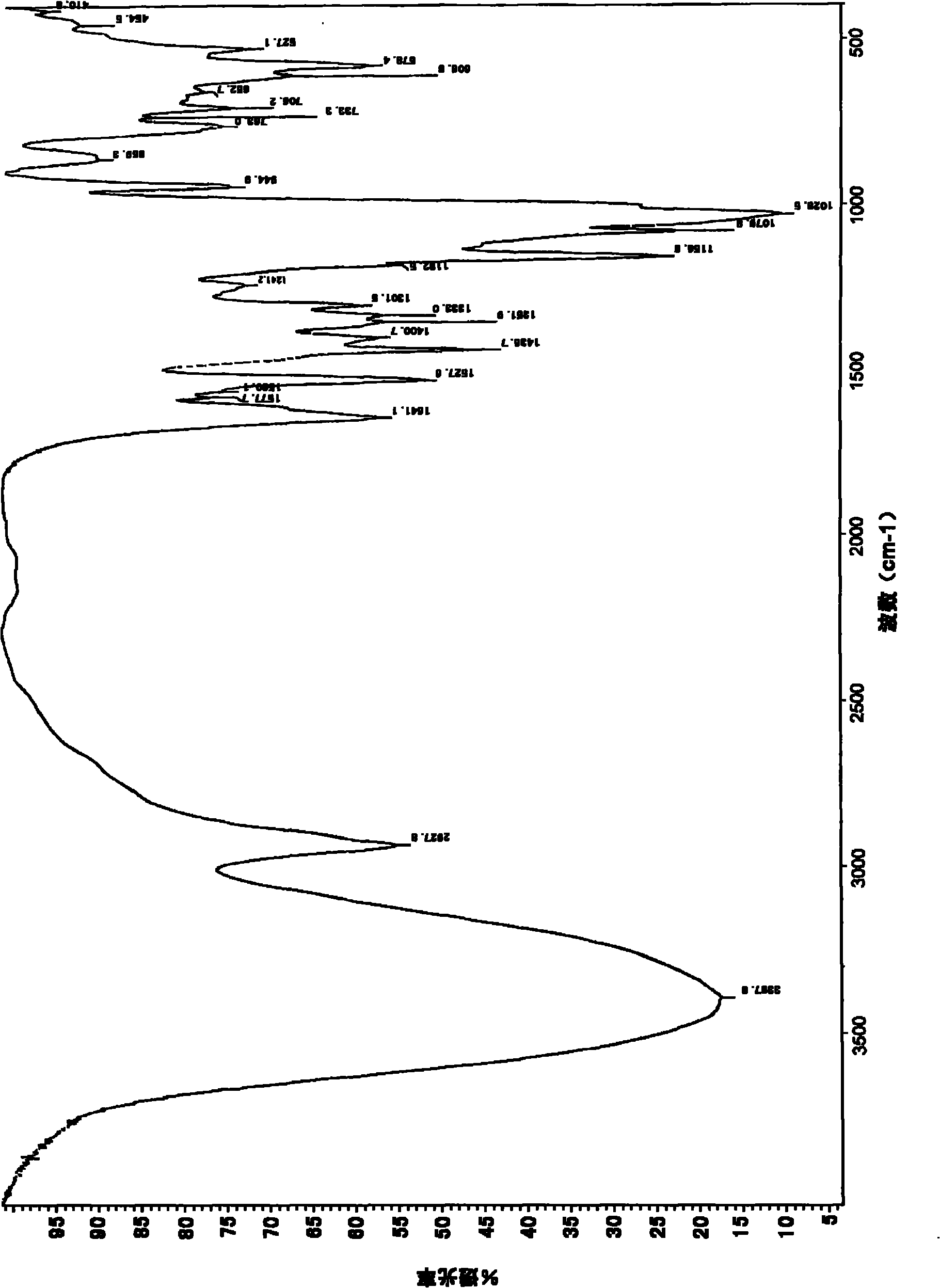
Image
Examples
Embodiment 1
[0023] Example 1 - Spray drying and vacuum drying were combined to prepare piroxicam β-cyclodextrin inclusion compound (molar ratio 1: 2.0-2.5).
[0024] Add 25g of β-cyclodextrin into a flask filled with 78ml of purified water, heat to 75°C, stir until dissolved, and adjust the pH value to 10.0 with 0.1M sodium hydroxide solution to obtain an aqueous solution of β-cyclodextrin. Take 3g of piroxicam and put it into a beaker, add 11.25ml of ethanol (concentration is 95%), stir to disperse evenly, and obtain a viscous solution. The viscous solution was added into an aqueous solution of β-cyclodextrin, and stirred at a constant temperature of 75° C. for 3 hours. Keep the temperature of the solution not lower than 70°C, and carry out spray drying, and the drying time is 20 minutes. The parameters of the spray dryer are as follows: the air flow of the blower (Blower) is 0.75m 3 / min, the atomizing pressure (Atomizing) is 15×10kPa, the inlet temperature (Inlet Temp) is greater tha...
Embodiment 2
[0031] Example 2 - Preparation of piroxicam β-cyclodextrin inclusion complex (molar ratio 1:2.0-2.5) by combining spray drying and vacuum drying.
[0032] Add 137g of β-cyclodextrin into a flask filled with 429ml of purified water, heat to 70°C, stir until dissolved, and adjust the pH value to 10.0 with 0.1M sodium hydroxide solution to obtain an aqueous solution of β-cyclodextrin. Take 16.5g of piroxicam and put it into a beaker, add 62ml of ethanol (concentration is 95%), stir to disperse evenly, and obtain a viscous solution. The viscous solution was added into an aqueous solution of β-cyclodextrin, and stirred at a constant temperature of 75° C. for 3 hours. Keep the temperature of the solution not lower than 75°C, and carry out spray drying, and the drying time is 31 minutes. The parameters of the spray dryer are as follows: The air flow of the blower (Blower) is 0.75m 3 / min, the atomizing pressure (Atomizing) is 15×10kPa, the inlet temperature (Inlet Temp) is greater ...
Embodiment 3
[0033] Example 3 - Preparation of tablets comprising piroxicam β-cyclodextrin inclusion compound
[0034] According to the following raw and auxiliary materials in each tablet prescription, tablets (1000 tablets in total) containing piroxicam β-cyclodextrin inclusion compound (prepared by Example 1) were prepared by direct compression method.
[0035] prescription:
[0036] Piroxicam β-cyclodextrin inclusion complex 200mg
[0037] Starch 187mg
[0038] Povidone (K30) 6.2mg
[0039] Talc 6mg
[0040] Sodium carboxymethyl starch 6.6mg
[0042]
[0043] Total 407.8mg (equivalent to piroxicam 20mg)
[0044] crafting process:
[0045] Pass 1000 times of the raw and auxiliary materials of the above prescription amount through a 100-mesh sieve, and fully mix until uniform (about 50 minutes). Dry tableting, that is, 1000 tablets.
[0046] The extruded tablet is according to " Chinese Pharmacopoeia " 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com