Patents
Literature
272 results about "INSECT BITES" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Foam and gel oat protein complex and method of use
InactiveUS6514487B1Smoothness and eleganceNormal skinCosmetic preparationsToilet preparationsAdditive ingredientIrritation
A composition containing enhanced colloidal oatmeal which utilizes other avena sativa ingredients to neutralize the discomfort, irritation and inflammation of the skin, as well as maintaining normal skin, and can be used to treat many types of discomforts, including itching; due to poison ivy, oak and sumac, insect bites, sunburn, chicken pox, hives, prickly heat, chafing, and the like while maintaining the normal pH of the skin.
Owner:BARR TERESA LEIGH
Method of producing an oil extract from seeds of plants via a binary azeotropic solvent mixture
The invention describes a method of producing extracts from the seeds of meadowfoam, brassicas and crambe plants. A number of subsidiary processes and steps are shown in order to extract differing fractions of oil. Products produced from the above method are also described including uses and methods of these products which include a variety of skin conditions including eczema, facial eczema, dermatitis, external ulcers, welts, rashes, insect bites, allergic reactions and other irritations, burns, wounds, psoriasis, acneiform eruptions, dryness, dry skin, irritation, skin atrophy, secondary infections and the like. The extracts are also described as being a useful compound for treatment of the symptoms of such skin conditions as described above. In particular the use and extraction of glucosinolate (GSL), thiocyanates (TCL) and isothiocyanates (ITCL) is described.
Owner:NEW ZEALAND BOTANICAL OILS
Methods and personal protection devices for repelling insects
InactiveUS7007861B2Low amountEliminate the problemBiocideLiquid surface applicatorsBillionthMosquito bite
Methods and devices for repelling insects are disclosed. The methods and devices provide personal protection from insect bites and insect landings, particularly mosquito bites. The methods and devices employ insect repellents such as pyrethroids at low (parts per billion) levels. The methods and devices effectively minimize the number of mosquitoes landing on a subject properly using the devices.
Owner:SC JOHNSON & SON INC
Mosquito repelling and itching relieving toilet water and preparation method thereof
ActiveCN103432051ALong effective timeEasy to prepareCosmetic preparationsBiocideBiotechnologyPropolis
The present invention relates to a mosquito repelling and itching relieving toilet water and a preparation method thereof, and belongs to the field of daily chemicals. The mosquito repelling and itching relieving toilet water is characterized by comprising the following raw materials, by weight: 2-4 parts of lavender essential oil, 6-10 parts of argy wormwood leaf oil, 5-7 parts of robust eucalyptus leaf oil, 10-15 parts of peppermint, 12-18 parts of wild dendranthema flower, 5-8 parts of propolis, 80-100 parts of deionized water, and 1-4 parts of an emulsifying agent, wherein the emulsifying agent is one or a plurality of materials selected from modified soybean phospholipid, glyceryl monostearate and glycerol monolaurate. According to the present invention, the mosquito repelling and itching relieving toilet water is prepared from the pure nature raw materials, has characteristics of mosquito repelling, itching relieving, long acting time and good insect bite dermatitis treatment effect, and is an ideal daily care product. In addition, the finished production preparation method is simple and easy to popularize and apply.
Owner:中山榄菊日化实业有限公司
Pharmaceutical composition for the treatment of itch
Disclosed is a topical preparation for the treatment of topical itch in humans and animals. The said composition consists of Opuntia, Propolis, Stearic Acid, Beeswax, Vegetable Oil and β-sitosterol. Itch includes scratch reaction itch, anal itch, or irritant itch due to plants (e.g., poison ivy), insect bite, sunburn, chemical itch, eczema, pruritis dermatitis, diabetic skin itch, aging skin itch, foot-itch, chickenpox, jock itch, hives, itch of healing burns and wounds, dry winter skin itch, and stress-related scalp itch, etc
Owner:PBN PHARMA
Interleukin-2 stimulated T lymphocyte cell death for the treatment of autoimmune diseases, allergic responses, and graft rejection
A method for the treatment or prevention of autoimmune diseases, allergic or atopic disorders, and graft rejection is provided, comprising inducing the death by apoptosis of a subpopulation of T lymphocytes that is capable of causing such diseases, while leaving substantially unaffected the majority of other T lymphocytes. Cell death is achieved by cycle(s) comprising challenging via immunization these T cells with antigenic substance at short time intervals, or by immunization followed by administering interleukin-2 (IL-2) when these T cells are expressing high levels of IL-2 receptor so as to cause these T cells to undergo apoptosis upon re-immunization with the antigenic peptide or protein. These methods are applicable to the treatment of autoimmune diseases such as, for example, multiple sclerosis, uveitis, arthritis, Type I insulin-dependent diabetes, Hashimoto's thyroiditis, Grave's thyroiditis, autoimmune myocarditis, etc., allergic disorders such as hay fever, extrinsic asthma, or insect bite and sting allergies, food and drug allergies, as well as for the treatment or prevention of graft rejection.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Skin care composition for dermatological disorders
InactiveUS20070190175A1Easy to synthesizeDecrease superficial wrinkleBiocideCosmetic preparationsWrinkle skinSkin texture
A topical skin care composition is provided to improve skin texture, diminish fine lines and wrinkles and decrease the appearance of hyper-pigmented areas. In addition, another embodiment of the skin care composition can be used to treat burns, insect bites and diminish the pain and scarring caused by such injuries.
Owner:TASKER PRODS IP HLDG CORP
Chinese-medicinal plaster for external wound
InactiveCN1657068AHeat-clearing and detoxifyingWith expelling wind and dampnessAntipyreticAerosol deliveryMentholBeriberi
A Chinese medicine in the form of ointment for treating trauma, cold injury, burn, scald, insect bite, eczema, sore, etc is prepared through frying 19 Chinese-medicinal materials including dandelion herb in boiling oil, filtering, adding the mixture of the powder prepared from 8 Chinese-medicinal materials including honeysuckle flower, etc and vaseline, heating, cooling and adding menthol.
Owner:葛宗训
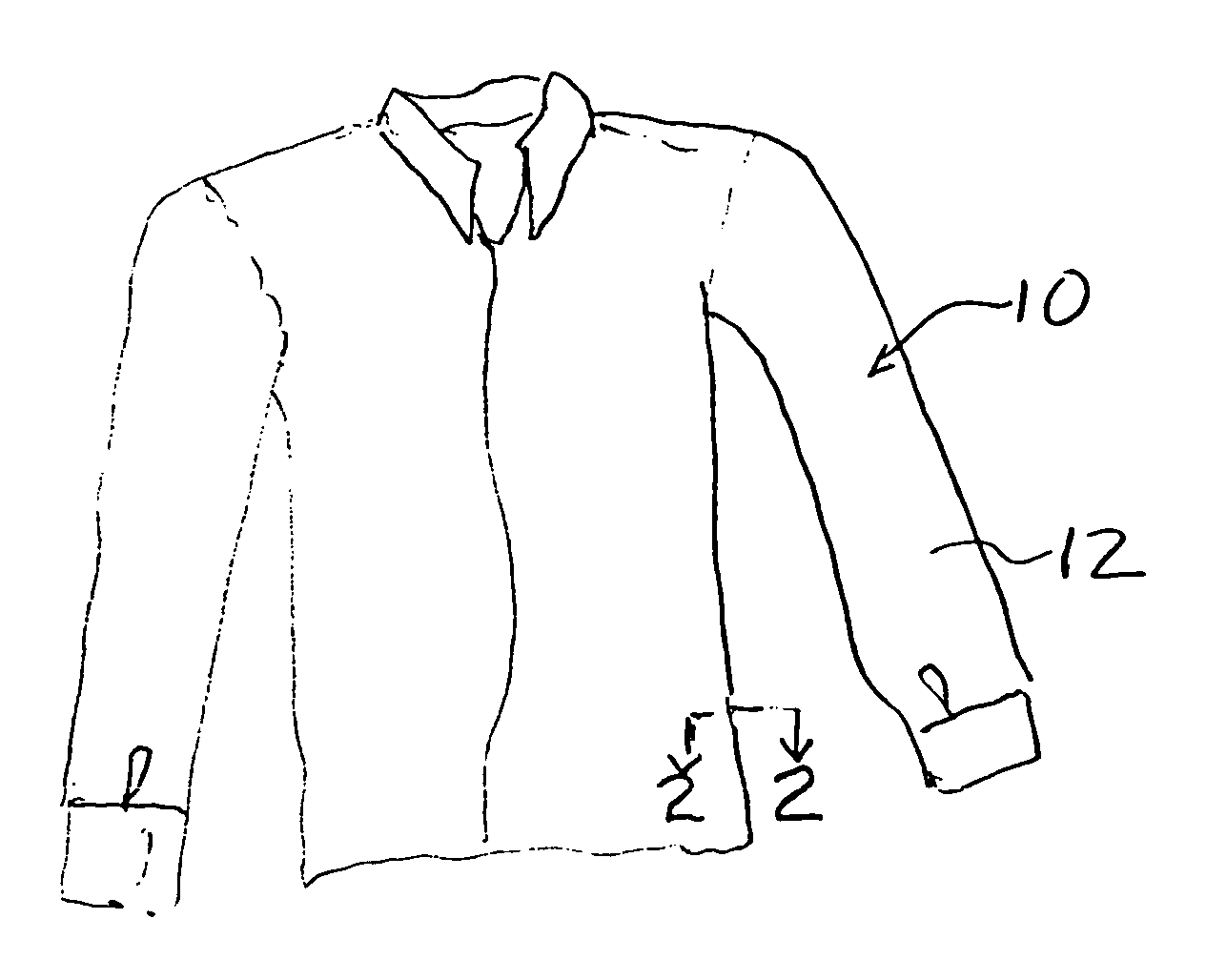
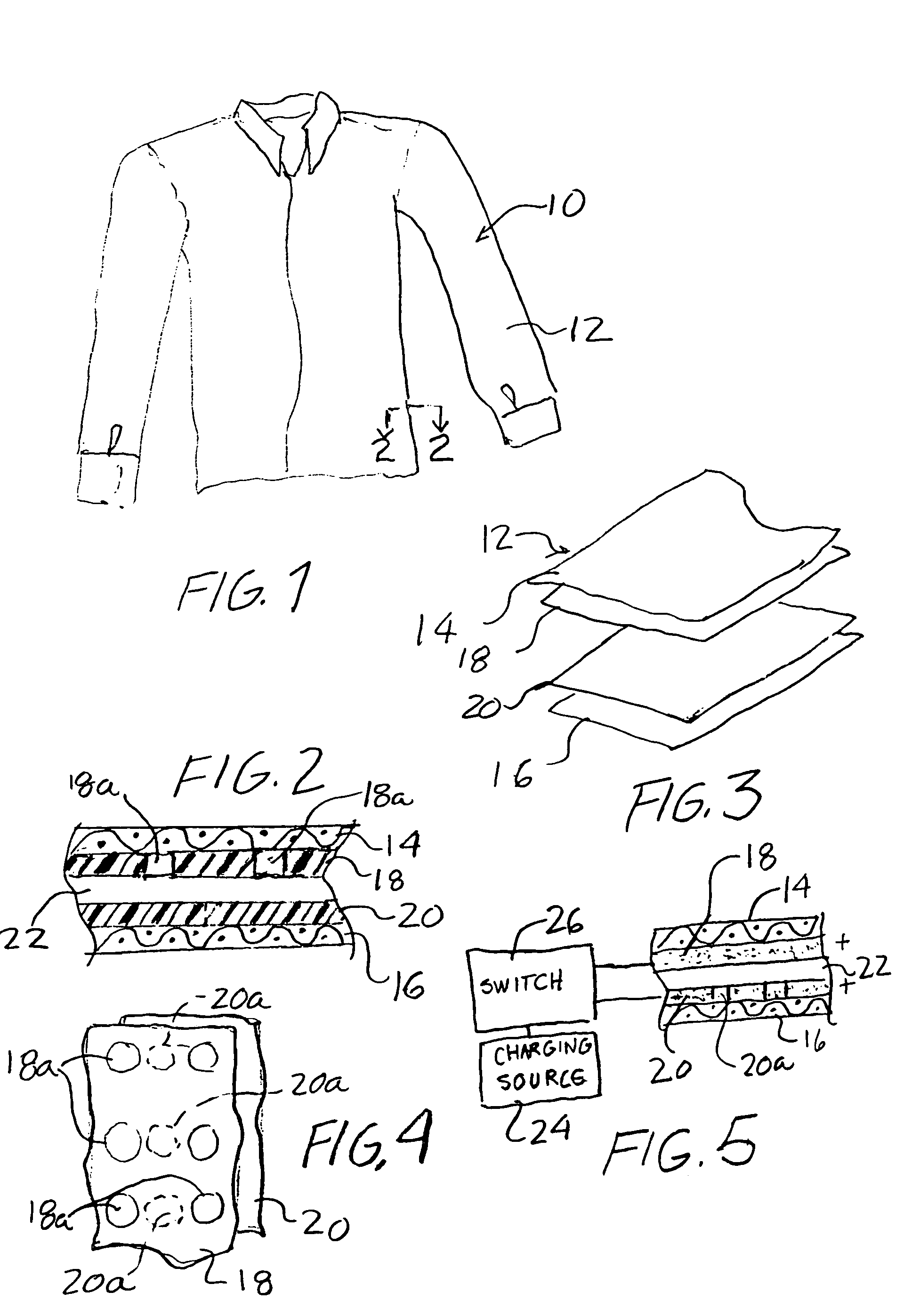
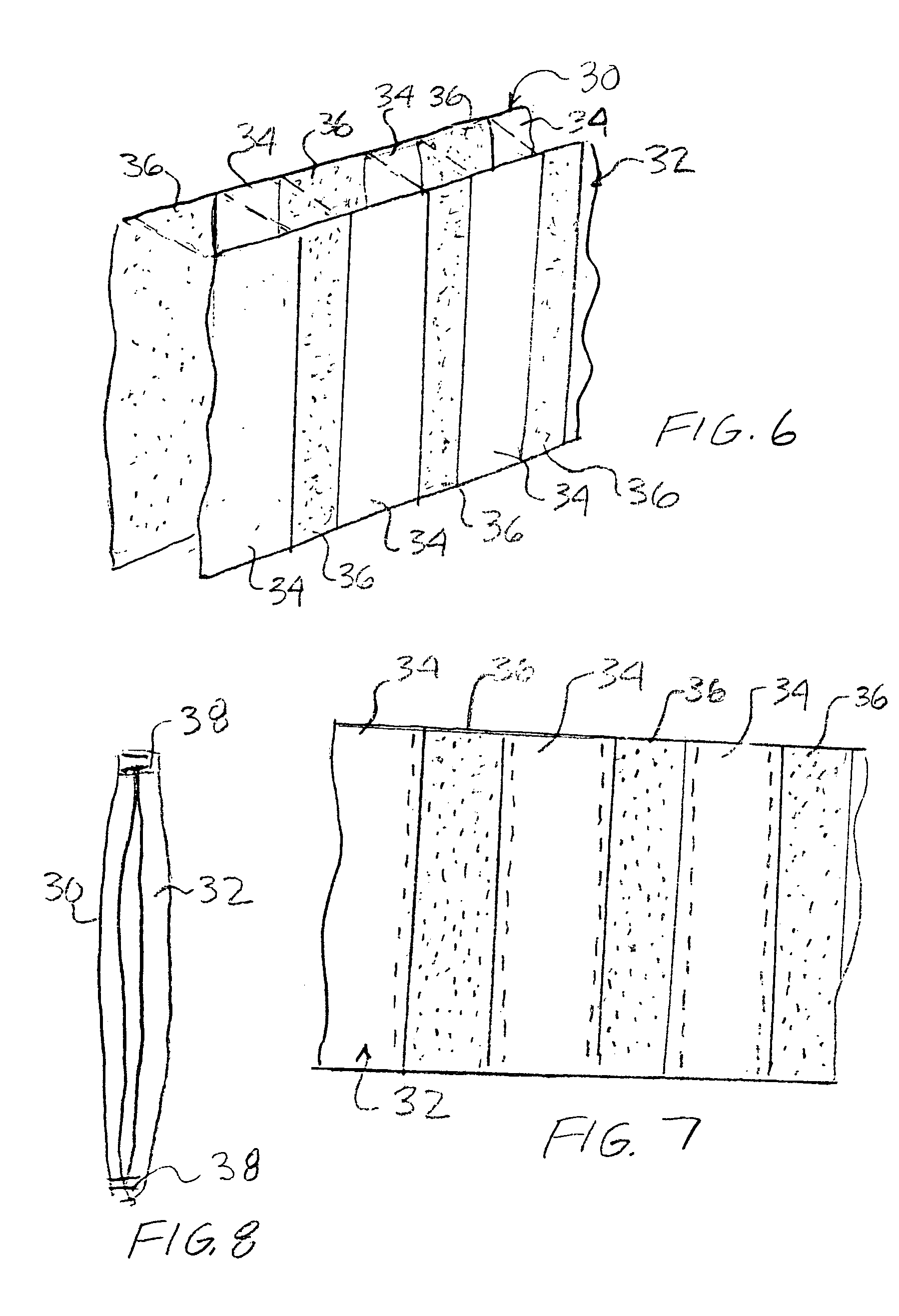
Breathable article of clothing that resists insect bites
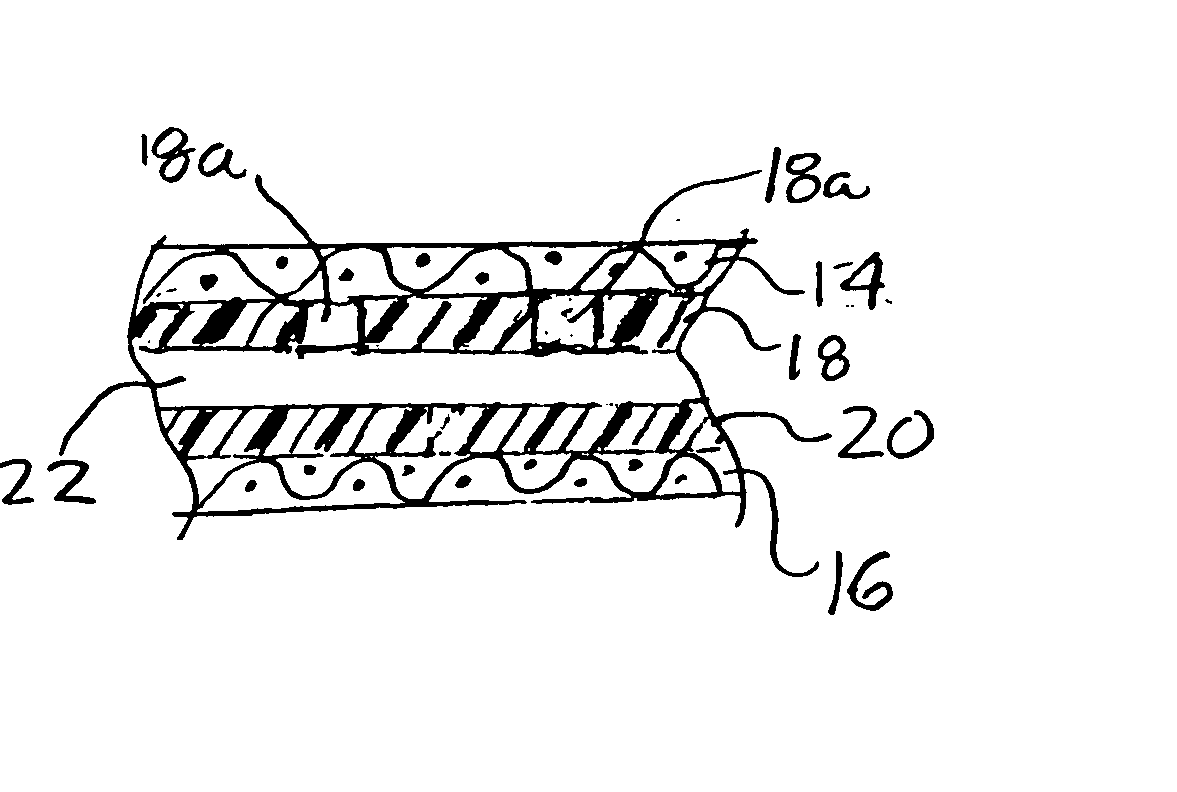
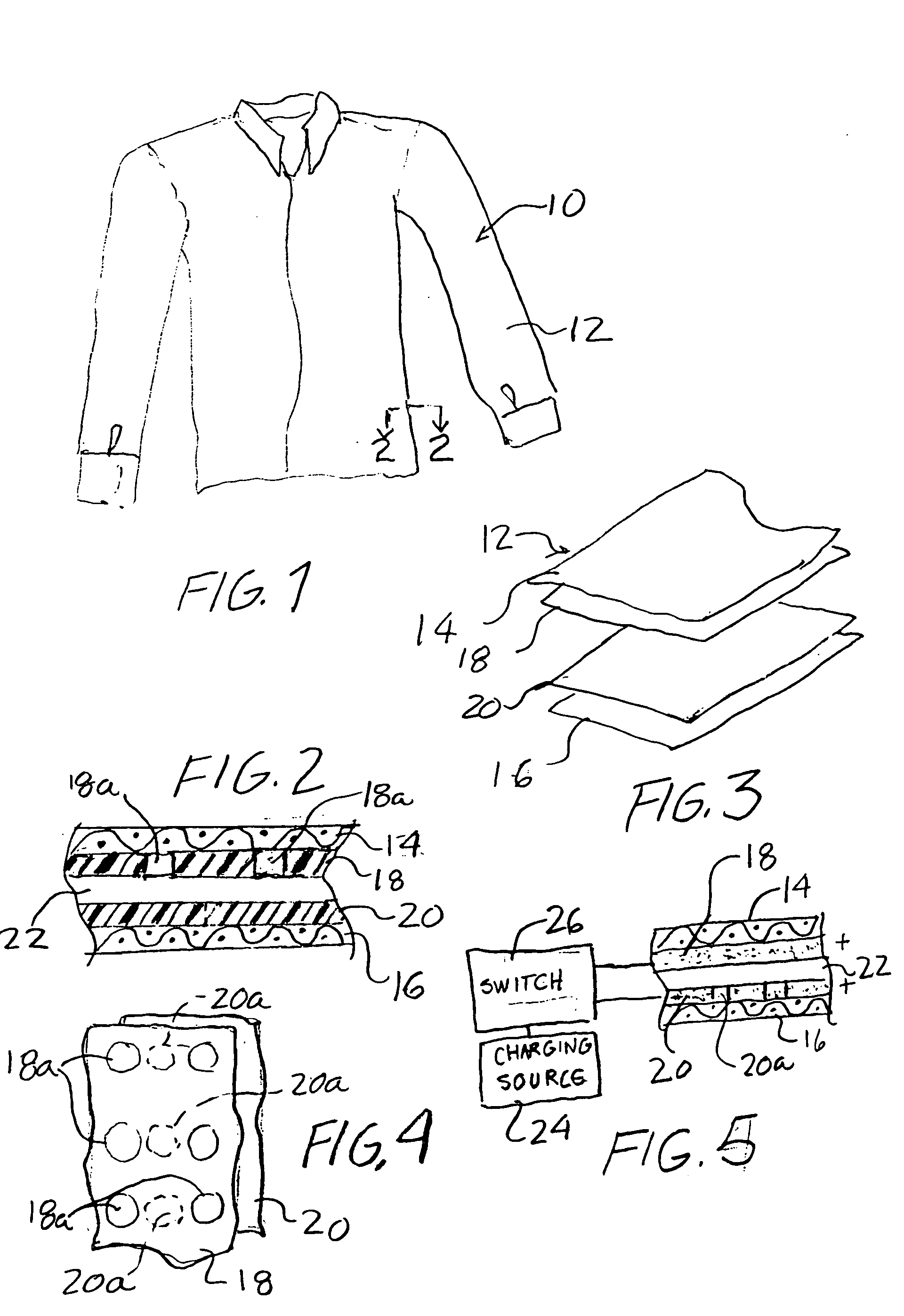
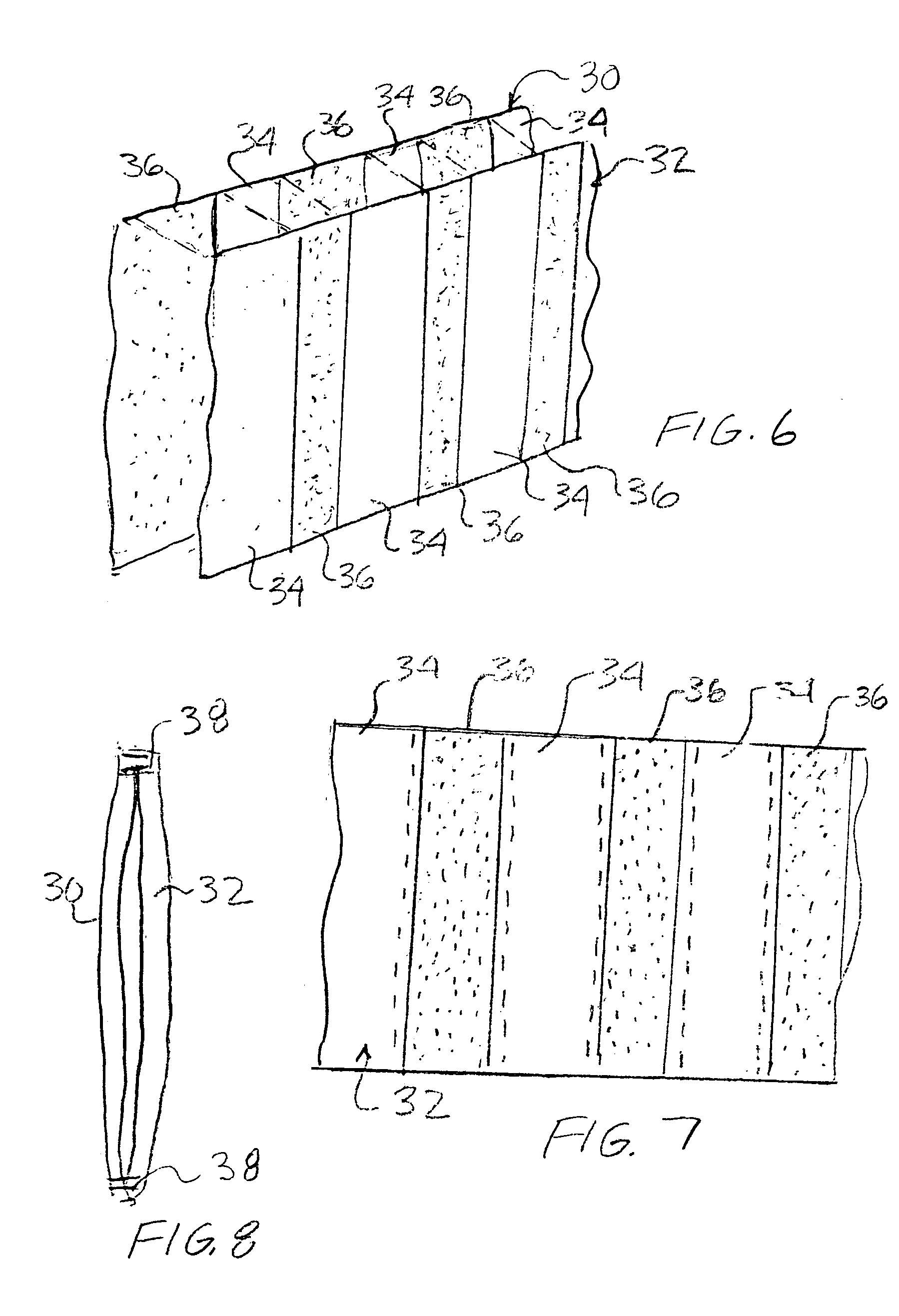
An article of clothing includes, in one embodiment, four alternate layers made of an air permeable material such as cotton and a non-air permeable material such as polyethyleneterephalate. Two intermediate layers both include a plurality of throughholes therein covered by the outer layers. The throughholes of the second layer are laterally offset from the throughholes of the third layer such that no direct path is provided through the throughholes between the intermediate layers so that the bites of insects are resisted. In another embodiment, two layers are provided each including a pattern, e.g., alternating stripes of an air permeable and a non-permeable material. The stripes of the two layers are offset so that non-permeable material of the two layers, taken together provide an insect barrier.
Owner:WALLERSTEIN ROBERT S
Analgesic composition for topical use
An analgesic composition, is disclosed which comprises a mixture of piroxicam, dexamethasone, ketamine, lidocaine injection, dimethyl sulfoxide, gabapentin and Vanicream™, preferably in the form of a cream or ointment. The composition is applied topically for the relief of pain of arthritis, neuropathy, post-herpetic (shingles) conditions, sore muscles, tendons and ligaments, and local reactions to insect bites or stings.
Owner:CATHCART CELEVATORON H
Topical base and active agent-containing compositions, and methods for improving and treating skin
InactiveUS20120100183A1Desirable tasteEasy to manageBiocideOrganic active ingredientsDiseaseIrritant dermatitis
The present invention provides unique, efficacious, inexpensive, safe, reliable, convenient, minimally bitter, skin protecting and penetrating, easy-to-administer base compositions and active agent-containing compositions, such as those including hydrocortisone, and related production and topical application methods, for treating the skin of mammals for a wide variety of different dermatologic conditions, disorders and diseases, such as inflammation, redness, cracking, insect bites, dryness, allergic reactions, trauma, irritant dermatitis, perleche, contact dermatitis, psoriasis, eczema, seborrheic dermatitis, acne excoriate, xerosis, eczema craquele, stasis dermatitis, disease related conditions and dryness from medications such as isotretinoin, acitretin and lipid lowering agents. This is effected by topically administering, or otherwise applying, effective amounts of the compositions thereto in forms that not only address the skin and mucosa of the mouth and lips, but also of the rest of the body and, in particular, areas where other topical balms containing hydrocortisone and other active ingredients have not been developed or marketed. Additionally, the flavoring addition to this product, and the base wherein the active ingredient(s) reside, affords a significantly better tasting, and less bitter, composition, thereby allowing a more pleasant experience and better compliance by patients. Larger sized stick formulation(s) allow for more applicability of the product, and more usefulness thereof, in various areas, and mucosal skin, of the body. The compositions include a unique formulation of FANCOL VB, Natunola Castor 1023, Finsolv TN, bees wax and, optionally, one or a plurality of plant or plant seed oils, fatty alcohols, fats and flavorings, in desirable weight percents thereof, in various forms, and preferably in the form of a solid roll-on stick present in a variety of sizes.
Owner:SCHLESSINGER JOEL +1
Medicine for treating dermatitis eczema, pruritus due to mosquito bites and use thereof
InactiveCN101361790ARaw materials are non-toxicLow priceHydroxy compound active ingredientsDermatological disorderCnidium monnieriTherapeutic effect
Owner:LOGISTICS UNIV OF CAPF
Composition
The invention describes a method of producing extracts from the seeds of meadowfoam, brassicas and crambe plants. A number of subsidiary processes and steps are shown in order to extract differing fractions of oil. Products produced from the above method are also described including uses and methods of these products which include a variety of skin conditions including eczema, facial eczema, dermatitis, external ulcers, welts, rashes, insect bites, allergic reactions and other irritations, burns, wounds, psoriasis, acneiform eruptions, dryness, dry skin, irritation, skin atrophy, secondary infections and the like. The extracts are also described as being a useful compound for treatment of the symptoms of such skin conditions as described above. In particular the use and extraction of glucosinolate (GSL), thiocyanates (TCL) and isothiocyanates (ITCL) is described.
Owner:NEW ZEALAND BOTANICAL OILS
Traditional Chinese medicine composition for treating eczema
InactiveCN104474076AImprove skin lesionsImprove itchingHydroxy compound active ingredientsPharmaceutical delivery mechanismToxic materialScabies
The invention relates to a traditional Chinese medicine composition for treating eczema. The traditional Chinese medicine composition is prepared from the following traditional Chinese raw medicines in parts by weight: 1-8 parts of wild chrysanthemum flower, 1-8 parts of senecio, 1-6 parts of encalyptus robusta, 1-8 parts of dandelion, 1-6 parts of radix sophorae flavescentis, 1-5 parts of mint, 1-6 parts of cortex dictamni, 1-8 parts of fructus kochiae, 1-8 parts of fructus cnidii and 1-2 parts of borneol. The composition is prepared into external powder or a washing lotion by a general medicine preparation method, or prepared into dosage forms for external application, such as emulsion and cream by being added with corresponding auxiliary materials. The traditional Chinese medicine composition has the main functions of clearing heat and toxic materials, dispelling wind, removing dampness and killing parasites to relieve itching, and can be used for effectively treating skin diseases such as dermatitis, eczema, furuncle, malignant boil, insect bite, scabies and skin infection.
Owner:CHONGQING TRADITIONAL CHINESE MEDICINE HOSPITAL
High-effective mosquito repelling liquid medicine
InactiveCN101700214AAnti-itchAnti-inflammatoryCosmetic preparationsToilet preparationsHypersensitive responseProtection Skin
The invention relates to high-effective mosquito repelling liquid medicine which is characterized by comprising the following components by weight percent: 2%-7% of N, N-Diethyl-m-toluamide, 0.2%-2% of glycyrrhiza extract (0.1%-90% of glycyrrhizinic acid or glycyrrhetinic acid or physiologically acceptable salt thereof), 0.2%-2% of flavescens extract (0%-90% of matrine or oxymatrine or physiologically acceptable salt thereof), 0%-50% of menthol or borneol and 0.01%-2% of plant essential oil. The high-effective mosquito repelling liquid medicine is the hormone-free preparation made of pure Chinese herbal medicine for external application and is prepared by uniquely combining the high-effective repellent with the components having the anti-inflammatory, the antibacterial and the antipruritic effect, thereby being innovative. The high-effective mosquito repelling liquid medicine of the invention has the function of repelling the mosquito, relieving the itching, refreshing the consciousness and protecting the skin from the insect bite and can repel the mosquito for 5-7 hours. The high-effective mosquito repelling liquid medicine of the invention is non-toxic to the skin and mucous membrane, can not cause the allergic reaction and has higher stability.
Owner:TIANJIN HEERBO BIOLOGICAL TECH CO LTD
Compositions and methods for an orally administered inhibitor of biting insects
InactiveUS20050008656A1Reduce inflammationReduce swellingBiocideAlgae medical ingredientsBenzoic acidPantothenic acid
The present disclosure concerns methods and compositions to inhibit insects from biting a subject. In preferred embodiments, the compositions may be administered orally, for example using a spray bottle to deliver to the mouth. In certain embodiments, the compositions and methods are effective to reduce swelling, itching, redness and / or inflammation of the local area of an insect bite. The compositions may include one or more herbs selected from the group consisting of rice bran, peppermint, barley grass, lobelia; chlorella, watercress, alfalfa and parsley and one or more vitamins selected from the group consisting of thiamin (B-1), riboflavin (B-2), niacin (B-3), pantothenic acid (B-5), pyridoxine (B-6), folic acid (B-9), cyanocobalamin (B-12), choline, inositol, d-biotin, para-aminobenzoic acid, and lecithin. Administration of effective amounts of the compositions is sufficient to inhibit insects from biting and / or treat insect affected areas of a subject.
Owner:MEREDITH SARAH
Compound chlorhexidine acetate spray
InactiveCN1557286AWide range of indicationsGood curative effectOrganic active ingredientsAerosol deliveryMentholChlorhexidine Acetate
The compound Chlorhexidinum acetate spray is prepared with Chlorhexidinum acetate, borneol, menthol, ethanol, essence and distilled water as material. Daily use and clinical observation shows that the spray may be used widely in treating various skin pruritus caused by different causes, including insect bite, irritability, eczema, etc. The present invention is one kind of safe, reliable, externally applied medicine with determined treating effect and low cost.
Owner:BEIJING KANGBIDE PHARMA
Topical therapeutic compositions containing bromelain
ActiveUS20150238576A1Suitable for topical applicationImmediate and long-lasting and effective reliefPeptide/protein ingredientsPharmaceutical delivery mechanismIrritationContact dermatitis
Compositions containing bromelain are disclosed for use as topical therapeutic agents to restore healthy skin, and for immediate and extended relief from itching and irritation associated with contact dermatitis, insect bites, idiopathic itch, chronic itch, hives, psoriasis, seborrhea, eczema and cracked fingertips, skin abrasions, cuts and minor burns as well as other indications. The compositions include lotions, creams and ointments.
Owner:KISS MY ITCH GOODBYE
External-use inflammation-diminishing itching-relieving ointment preparation and preparation method thereof
InactiveCN102861271AReasonable compositionMany indicationsDermatological disorderAluminium/calcium/magnesium active ingredientsSolar dermatitisRubella
The invention provides an external-use inflammation-diminishing itching-relieving ointment preparation and a preparation method thereof and belongs to the technical field of traditional Chinese medicine preparation. The external-use inflammation-diminishing itching-relieving ointment preparation is prepared by natural traditional Chinese medicines: 20-50 parts of radix sophorae flavescentis, 10-20 parts of fructus cnidii, 20-30 parts of fructus kochiae, 20-30 parts of golden cypress, 20-30 parts of rhizoma atractylodis, 20-40 parts of rhizoma smilacis glabrae, 10-20 parts of dried alum and a proper amount of menthol crystal. According to clinical study and experiment results, the external-use inflammation-diminishing itching-relieving ointment preparation has the effect of clearing heat and eliminating dampness, dispelling wind and arresting itching, detoxifying and removing stasis and can be used for treating symptoms of pruritus, red and swollen of skin, exudation and the like caused by eczema, rubella, urticaria, solar dermatitis, insect bite and the like. The external-use inflammation-diminishing itching-relieving ointment preparation has certain effect of relieving symptoms of patients, alleviating pruritus and pain and the like, causes no obvious adverse reaction, and is high in safety, wide in raw material source and low in production cost.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
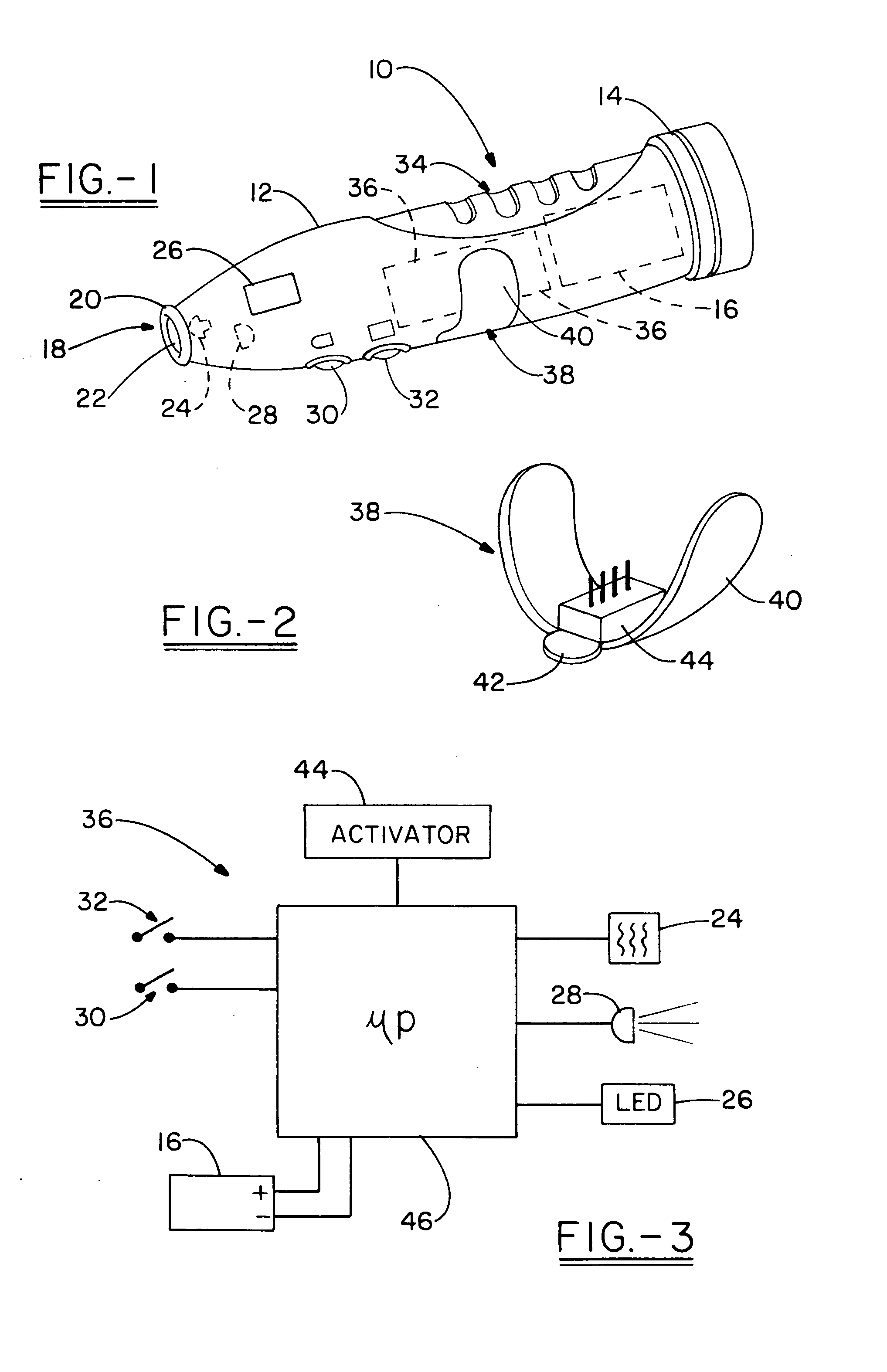
Therapeutic treatment device and method for cold sores and the like
A therapeutic treatment device for treating colds sores and the like. The device includes a housing configured to be hand held, and having an annular pad at one end thereof configured to engage and area of the body of the user. A heat source is maintained adjacent the pad. A controller, in the form of a programmable chip or the like, allows for the heat source to be energized for a particular period of time, shutting off automatically. After a predetermined quiescent period of time has expired, the device signals the user that the heat source can be energized again, with the controller then maintaing the activation of the heat source for a second preselected period of time. The cycle continues until the treatment is complete. A treatment actuator communicates with the controller to enable the controller to process through the treatment sequence. The treatment activator may be specifically tailored to any of various treatments, including the treatment for a cold sore, an insect bite, a bee sting, a spider bite, or the like.
Owner:THE JENEX CORP
Medicine composition containing matrine class alkaloid, preparation method and pharmaceutical application
The invention provides a medicine composition containing matrine class alkaloid, a preparation method and pharmaceutical applications. The medicine composition is the combination of the matrine class alkaloid and inflammation-resisting pain-relieving class medicines. The inflammation-resisting pain-relieving medicine comprises the non-steroidal inflammation-resisting medicines of aspirin, acetaminophen, indometacin, ibuprofen, oxyphenbutazone, naproxen, mefenamic acid, diclofenac sodium, celecoxib, rofecoxib, valdecoxib and the like and also comprises the vegetable inflammation-resisting pain-relieving class medicines of escin, ferulic acid, berberine, wilfordine, ephedrine and the like and the pain-relieving class medicines of morphia, demerol and the like. The matrine class alkaloid and one or various of the inflammation-resisting pain-relieving class medicines can form a medicine composition used for the pharmaceutical applications of resisting cold, allaying a fever and treating the swelling and pain of the bone joint and the muscle, rheumatic diseases, cardiovascular diseases, arteriosclerotic diseases, tumors, anaphylactic diseases, senile dementia, mosquito bite, insect bite and the like.
Owner:QINGDAO QIYUAN BIO TECH CO LTD
Topical application of melatonin directly or in liposomes for the amelioration of itching and histamine and non-histamine related inflammatory skin changes
The invention is a topical therapeutically effective amount of melatonin encapsulated in a liposome applied topically to an area of skin affected by immunologic response, radiation treatment induced dermatitis, acne, insect bite or other irritant stimulus such as sunburn in order to reduce itching. The invention also proposes a topical therapeutically effective amount of melatonin and reduced glutathione encapsulated in a liposome applied topically to an area of skin for relief of those afflictions.
Owner:GUILFORD F TIMOTHY
Lavender perfume
InactiveCN104644479AFragrant and elegantImprove permeabilityCosmetic preparationsToilet preparationsCuticleMoisture
Owner:AGATE PERFUME MINGGUANG
Compound essential oil for expelling mosquito
A compound essential oil for expelling mosquito comprises the following plant essential oils: by volume, 50-100 parts of citronella essential oil, 20-50 parts of rosemary essential oil, 10-20 parts of lemon essential oil, 15-30 parts of tea tree essential oil, 5-10 parts of pine needle essential oil, 1-2 parts of thyme essential oil and 5-10 parts of juniper berry essential oil. The compound essential oil is an excellent pest repellant using the aromatic plant essential oils in nature. All essential oil producing plants have natural defense systems against foreign predators. The essential oils secreted out from smiling oil glands of leaves can protect, for thousands of years, the plants from insect bite. Now people can extract the essential oils, and use the essential oils to expel insects.
Owner:SUZHOU NATESU BIOLOGICAL SCI & TECH
Breathable article of clothing that resists insect bites
InactiveUS20050278837A1Moderate to light weightChemical protectionHeat protectionPolyethylene terephthalateInter layer
An article of clothing includes, in one embodiment, four alternate layers made of (i) an air permeable material such as cotton and (ii) a non-air permeable material such as polyethyleneterephalate. Two intermediate layers both include a plurality of throughholes therein covered by the outer layers. The throughholes of the second layer are laterally offset from the throughholes of the third layer such that no direct path is provided through the throughholes between the intermediate layers so that the bites of insects are resisted. In another embodiment, two layers are provided each including a pattern, e.g., alternating stripes of an air permeable and a non-permeable material. The stripes of the two layers are offset so that non-permeable material of the two layers, taken together provide an insect barrier.
Owner:WALLERSTEIN ROBERT S
Sore ulcer treating ointment and its prepn
InactiveCN1403097AEasy accessImprove absorption rateInanimate material medical ingredientsMammal material medical ingredientsDiseaseWhite petrolatum
The present invention discloses one kind of sore ulcer treating ointment and its preparation process. The medicine is prepared with liquid paraffin, calamine, borneol, pearl, borax and other six kinds of Chinese medicinal materials and through crushing, mixing and other steps. The present invention has simple preparation process, low cost and high treating effect on pus pocket, boil, carbuncle, ulcer, insect bite and other skin diseases.
Owner:MAYINGLONG PHARMA GROUP
Disinfecting pain relieving heat dispersing spirit raising medicinal liquid
A Chinese medicine in the form of interior-taken or exterior-taken liquid for treating cold, stomachache, diarrhea, vomiting, insect bite, dizziness, indigestion, burn, scald, etc disinfecting, detoxi cating and refreshing is prepared from 6 Chinese-medicinal materials including edible alcohol, menthol, plum, etc. Its preparing process is also disclosed.
Owner:林蔼煌
Methods and personal protection devices for repelling insects
InactiveUS20060226249A1Low amountEffectively repelledBiocideSpray nozzlesChrysanthemum cinerariifoliumZoology
Methods and devices for repelling insects are disclosed. The methods and devices provide personal protection from insect bites and insect landings, particularly mosquito bites. The methods and devices employ insect repellents such as pyrethroids at low (parts per billion) levels. The methods and devices effectively minimize the number of mosquitoes landing on a subject properly using the devices.
Owner:SC JOHNSON & SON INC
External use drug for quickly relieving itching, eliminating swelling and rash, and resisting mosquito and insect bites
InactiveCN104983674AEliminate rednessEliminate rashAntibacterial agentsHydroxy compound active ingredientsHuman bodySide effect
The invention discloses an external use drug for quickly relieving itching, eliminating swelling and rash, and resisting mosquito and insect bites. The external use drug is prepared by mixing the following raw materials in parts by weight: 10 to 150 parts of menthol, 10 to 150 parts of borneol, 5 to 30 parts of ethylparaben, 40-300 parts of natural gel, 500-1000 parts of ethanol, 200-500 parts of glycerin and 0-500 parts of pure water. According to the external use drug provided by the invention, the natural gel is used as a carrier, the natural menthol is used as the main raw material, some natural polymeric substances are matched, and a percutaneous absorption process is adopted to enable the active ingredients to penetrate the skin, so that the effects of relieving itching and eliminating swelling and rash are achieved; the manner of applying the drug externally is adopted; after the drug is applied to the meridian points of human bodies, the active ingredients run with meridian circulation, and the effects of removing heat and resisting mosquito and insect bites are remarkable; in addition, the drug does not enter the digestive system of human bodies, therefore, no harm or toxic or side effect is caused; the external use drug is a safe, and environment-friendly product with the functions of relieving itching, eliminating swelling and rash, and resisting mosquito and insect bites.
Owner:WUHAN LANGQI BIOLOGICAL SCI & TECH
Manual sowing tool
InactiveCN106416525APrevent mildewImprove germination rateHand sowing implementsEngineeringUltimate tensile strength
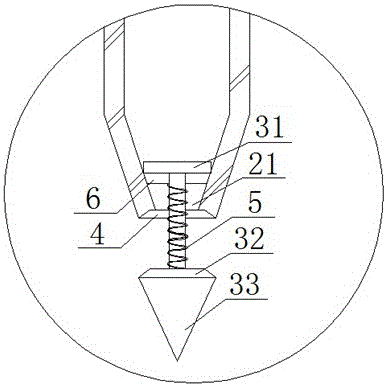
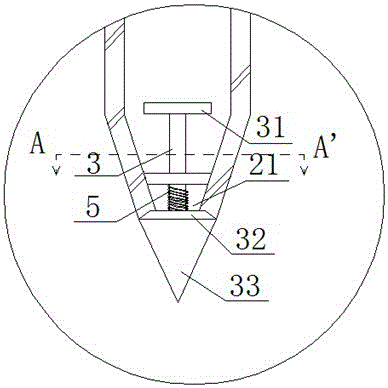
A manual sowing tool is characterized by comprising a storage barrel, wherein the storage barrel comprises an upper end cover, a barrel body and an inner storage cavity defined by the upper end cover, a lower end surface and the barrel body, a handheld handle is arranged on the upper end cover, and a drying agent is arranged in the upper end cover; a discharging hole is formed in the lower end surface, a propping rod capable of sliding in the discharging hole up and down is arranged in the discharging hole, a first sealing plate is arranged at one end, located in the storage cavity, of the propping rod, and a second sealing plate is arranged at the other end; a limiting rack is fixed in the discharging hole and arranged on the propping rod in a sleeving manner. Therefore, vegetable seeds can be stored in the inner storage cavity and subjected to drying storage by the aid of the drying agent in the upper end cover, so that the defect that the seeds after affected with damp are susceptible to mildew or insect bites, and the germination rate of the seeds is increased; during sowing, a user holds the handheld handle and can bury the seeds in soil through the propping rod without bowing, the labor intensity is reduced, and the sowing efficiency is improved.
Owner:FOSHAN TIANSHUN TECH CO LTD
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com