Triglyceride prodrug based on lymphatic mediated transport and preparation method thereof
A prodrug and pharmaceutical technology, applied in the field of medicine, can solve the problems of few researches and systematic discussions without researches
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
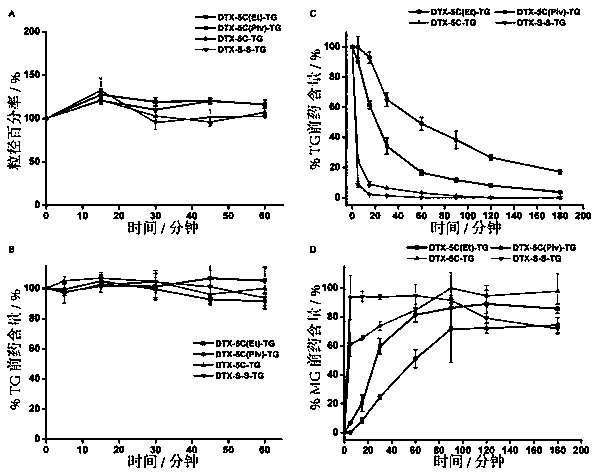
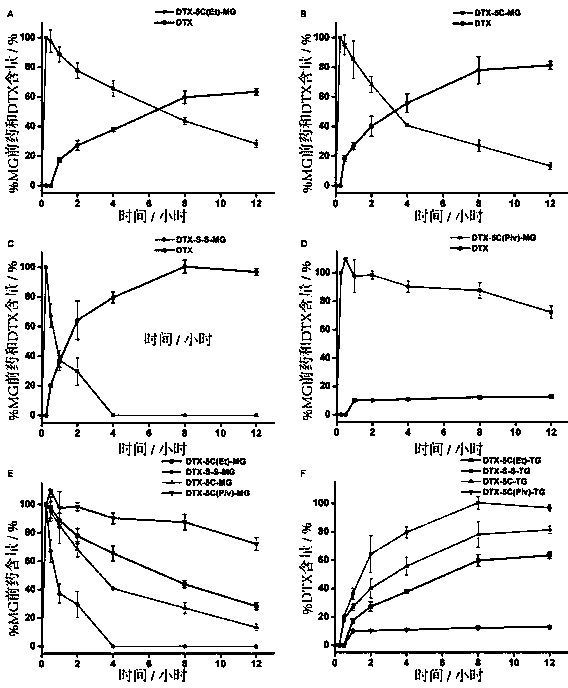
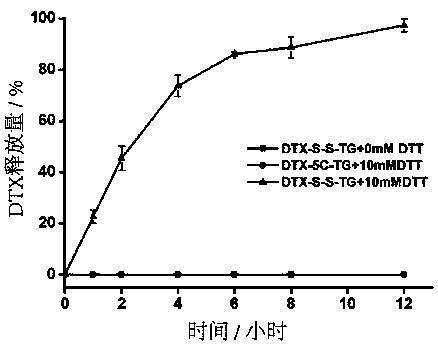
Image
Examples
example 1
[0079] Example 1 Preparation of docetaxel-C5-triglyceride prodrug (DTX-5C-TG)
[0080] The structure is as follows
[0081]
[0082] Dissolve 10.25g (40mmol) of palmitic acid in 100ml of anhydrous dioxane, add 3 drops of N,N-dimethylformamide, add 4.9g (41mmol) of thionyl chloride dropwise, and vacuumize under nitrogen protection after dropping. Reflux at 110°C for 4h, and concentrate the reaction solution under reduced pressure to obtain a pale yellow oil. Dissolve 1.75g (20mmol) of 1,3-dihydroxyacetone in 100ml of anhydrous dichloromethane, add 5ml (40mmol) of triethylamine, quickly add the above oily substance dropwise under ice bath, vacuumize nitrogen protection after dropping, at room temperature React overnight. The reaction solution was concentrated, washed twice with distilled water, the aqueous layer was extracted with chloroform, the organic layer was washed once with saturated brine, the organic layer was dried over anhydrous sodium sulfate, and the solvent ...
example 2
[0085] Example 2 Preparation of docetaxel-C5(Et)-triglyceride prodrug (DTX-5C(Et)-TG)
[0086] The structure is as follows
[0087]
[0088] The preparation method of 1,3-dipalmitin is the same as example 1. Dissolve 1.5g (6.33mmol) of L-glutamic acid-γ-Benyl ester in 10ml of acetic acid, add 10ml of water, stir in ice bath for 20min, add 3.45g (50mmol) of sodium nitrite, react in ice bath for 2h, and react overnight at room temperature . The reaction solution was extracted with ethyl acetate, the organic layer was washed once with water, the organic layers were combined, dried over anhydrous sodium sulfate, and the solvent was removed by rotary evaporation to obtain compound 2. 1 g of compound 1 was redissolved in 30 ml of anhydrous dichloromethane, 100 mg (0.8 mmol) of DMAP was added, and 0.6 g (5.88 mmol) of acetic anhydride was added dropwise. After the dropping, the mixture was vacuumed under nitrogen protection and reacted overnight at room temperature. The solvent w...
example 3
[0090] Example 3 Preparation of docetaxel-C5(Piv)-triglyceride prodrug (DTX-5C-(Piv)-TG)
[0091] The structure is as follows
[0092]
[0093] The preparation method of 1,3-dipalmitin is the same as example 1. Dissolve 1.5g (6.33mmol) of L-glutamic acid-γ-Benyl ester in 10ml of acetic acid, add 10ml of water, stir in ice bath for 20min, add 3.45g (50mmol) of sodium nitrite, react in ice bath for 2h, and react overnight at room temperature . The reaction solution was extracted with ethyl acetate, the organic layer was washed once with water, the organic layers were combined, dried over anhydrous sodium sulfate, and the solvent was removed by rotary evaporation to obtain compound 2. 1 g of compound 1 was redissolved in 30 ml of anhydrous dichloromethane, and 100 mg of (0.8mmol) DMAP, 770mg (6.28mmol) of pivaloyl chloride was added dropwise, and after the dropwise evacuation under nitrogen protection, the reaction was carried out overnight at room temperature. The solvent ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| electric potential / voltage | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com