Application of cyclodextrin-methyl vinyl ether/maleic anhydride copolymer and its self-assembled nanoparticles in oral drug delivery
A technology of methyl vinyl ether and maleic anhydride, which is applied in the direction of non-active ingredients of polymer compounds and powder delivery, can solve the problems of limited solubilization ability and poor transmembrane absorption ability of insoluble drugs, and achieve improved oral Bioavailability, reduced first-pass effect, and enhanced bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
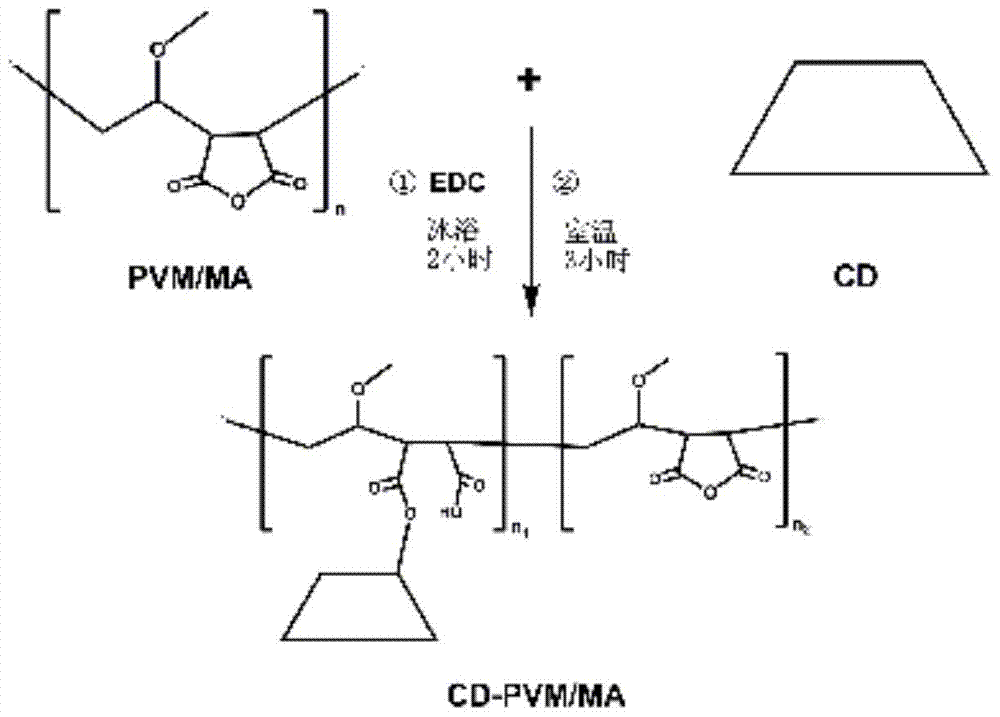
[0033] CD-PVM / MA was synthesized from hydroxypropyl β-cyclodextrin (HP-β-CD) and PVM / MA (molecular weight: 200,000).
[0034] Dissolve PVM / MA and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) in acetone / tetrahydrofuran (3 / 2, v / v), ice bath After activating for 2 hours, add HP-β-CD (36 times the molar weight of PVM / MA), react at room temperature (25°C) and nitrogen protection for 3 hours, and obtain a white powder after separation and purification, which is CD-PVM / MA. The reaction formula is as follows:
[0035]
Embodiment 2
[0037] The molecular weight of PVM / MA in the reaction formula ranges from 100,000 to 2.5 million, and the cyclodextrin can be α, β, γ cyclodextrin and their derivatives.
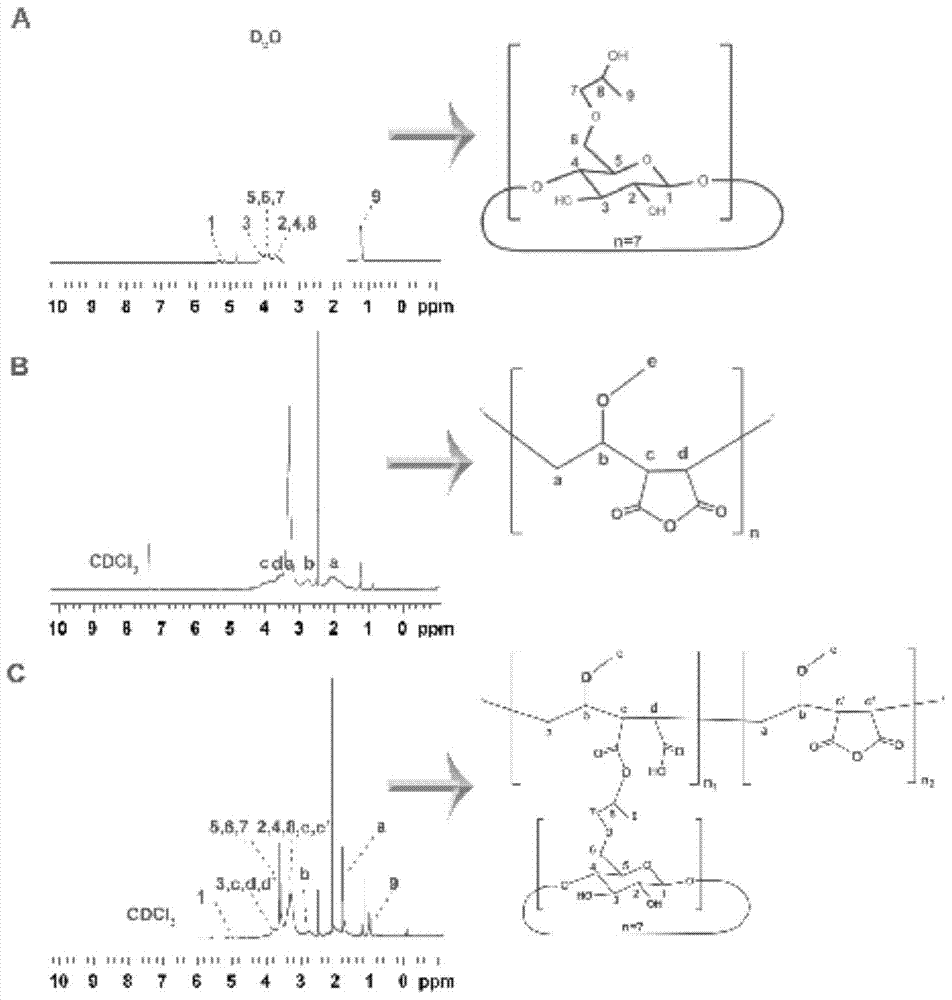
[0038] Determination by NMR 1 HNMR hydrogen spectrum to determine the structure of CD-PVM / MA in embodiment 1, PVM / MA and CD-PVM / MA are solvent with deuterium band dichloromethane, HP-β-CD is solvent with heavy water, the results see figure 2 . The signal peak of CD is attributed to 3.5-4.0ppm (hydroxyglucose ring C3, 4, 5, 6 methine); the PVM / MA signal peak is assigned as follows: 3.567ppm (methine on the parent chain), 2.513ppm ( ester group), 2.181 ppm (hydrogen on cyclic anhydride). Compared with the NMR spectrum of PVM / MA, the NMR spectrum of cyclodextrin-grafted PVM / MA has a split peak (signal peak of hydroxyl cyclodextrin) at 3.5-4.0ppm, and the NMR spectrum shifts to the high field, indicating that cyclodextrin Grafted onto PVM / MA.
Embodiment 3
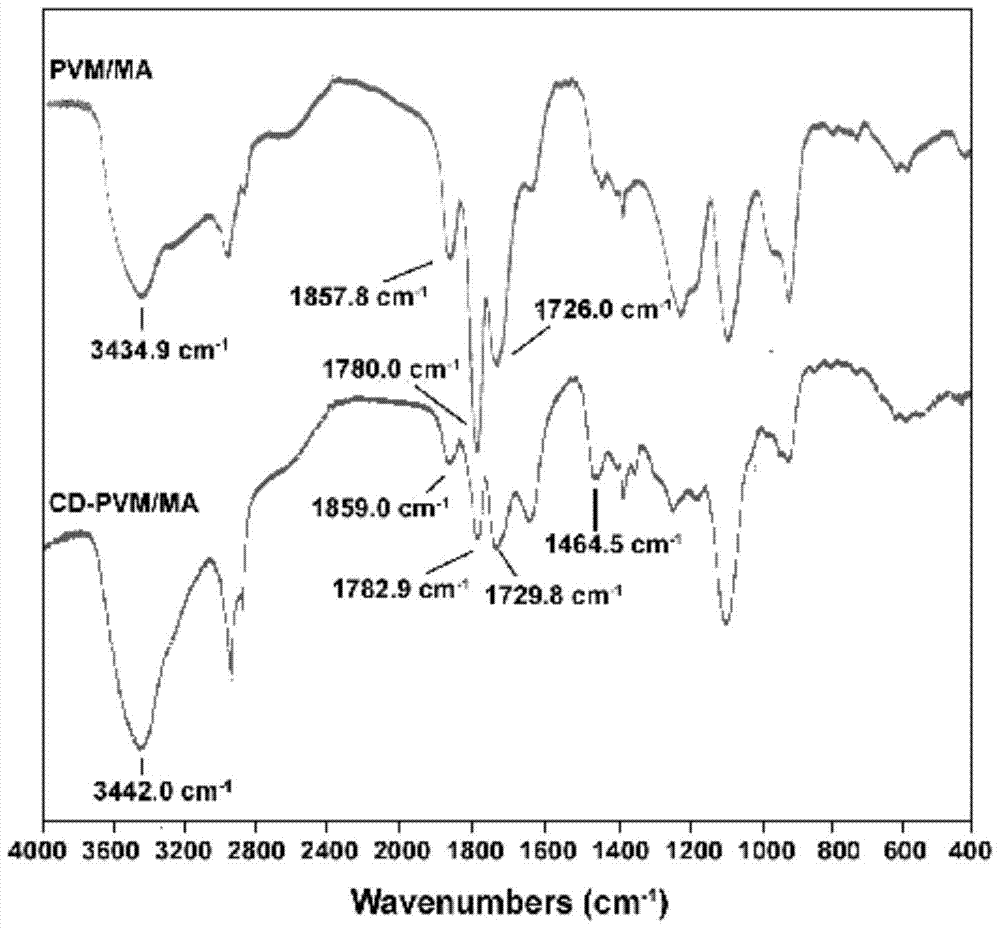
[0040] Adopt infrared spectrum to determine the structure of CD-PVM / MA in embodiment 1, select KBr to be blank auxiliary material for use, the result is as follows image 3 . Comparing the infrared spectra of CD-PVM / MA and PVM / MA, it can be seen that the position and intensity of the characteristic peaks of the infrared spectra changed significantly before and after cyclodextrin was grafted on PVM / MA. The peaks at 1782, 1729, and 1250 become blunt and the signal weakens (cyclic anhydride C=O signal peak, the number of cyclic anhydrides decreases). Since the two compounds are high molecular weight polymers, the hydrolysis of cyclic anhydride and the grafting of cyclodextrin are incomplete, so some characteristic peaks of PVM / MA still remain in the infrared spectrum of CD-PVM / MA. Infrared spectra showed that cyclodextrin was successfully grafted onto PVM / MA, and the grafting rate was high.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com