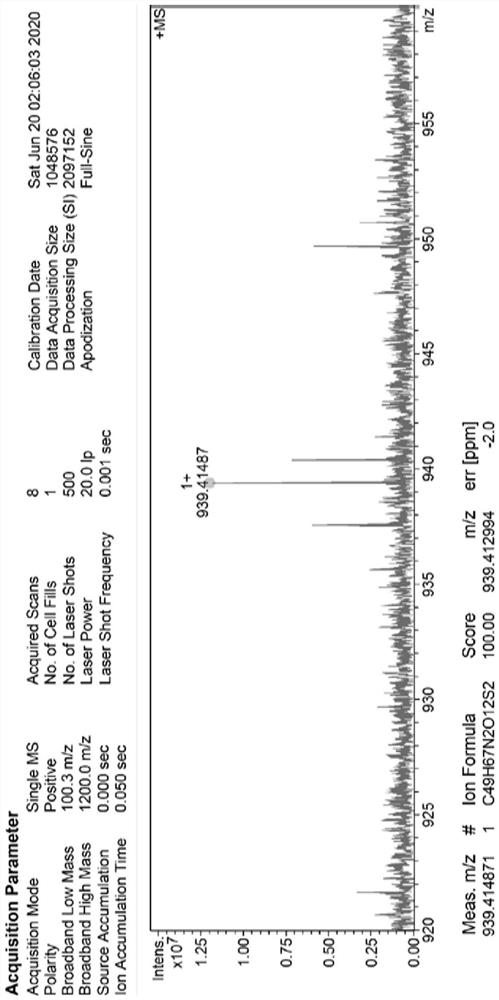
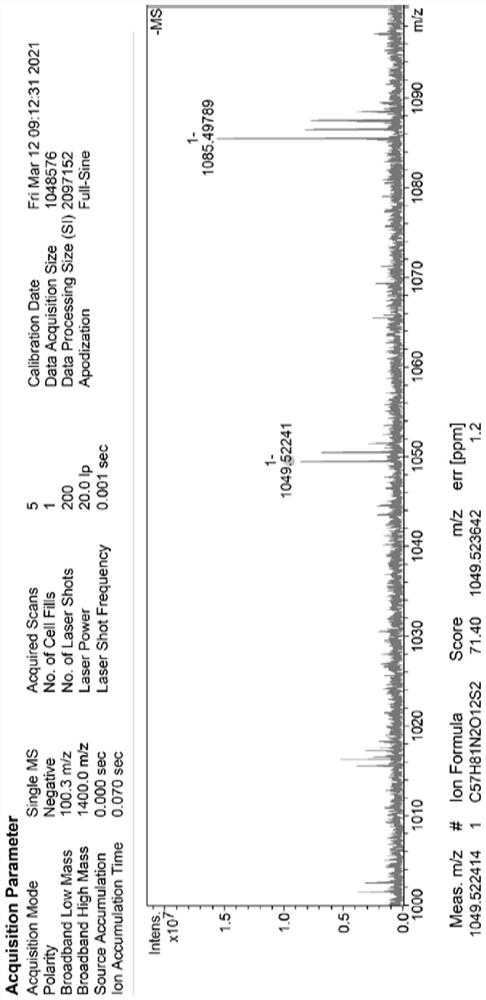
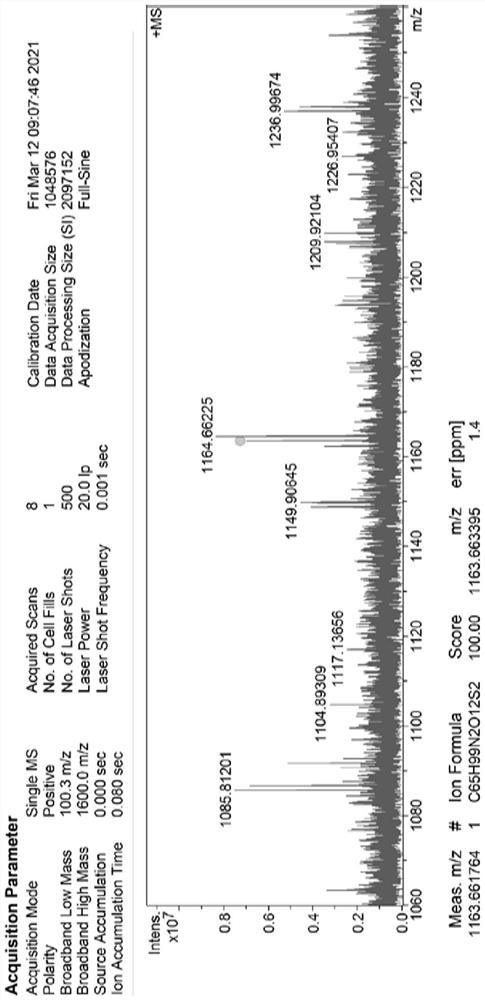
SN38 triglyceride prodrug, lipid preparation as well as preparation method and application of SN38 triglyceride prodrug and lipid preparation
A technology of triglycerides and prodrugs, which is applied in the field of medicine, can solve problems such as side reactions, and achieve the effects of strong repeatability, enhanced stability, and uniform and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation method of the SN38 class triglyceride prodrug (SN38-SS-OcA) substituted by octanoic acid group comprises the following steps:
[0048] The structure of the prodrug is as follows:
[0049]
[0050] (1) Synthesis of 1,3-diglyceride (1,3-DG): 2.5g (17.34mmol) octanoic acid was dissolved in 50ml dichloromethane, and 5.2g (27.13mmol) EDCI and 1.1g (9mmol) Under the catalysis of DMAP, react with 0.65g (7.22mmol) 1,3-dihydroxyacetone to form an ester, and then react with 0.2g (5.28mmol) sodium borohydride for hydrogenation to obtain 1,3-DG.
[0051] (2) Dissolve 0.7g (2.03mmol) of 4,4'-dithiodibutyric acid in 4ml of acetic anhydride, react at room temperature for 2h, concentrate by rotary evaporation and redissolve in 30ml of anhydrous dichloromethane, And added 0.68g (1.98mmol) 1,3-DG, 22.2mg (0.2mmol) DMAP. Vacuum nitrogen protection at room temperature for 12h. The solvent was removed by rotary evaporation, and the intermediate product was obtained by ...
Embodiment 2
[0057] The preparation method of the SN38-class triglyceride prodrug (SN38-SS-LA) substituted by lauric acid group may further comprise the steps:
[0058] The structure of the prodrug is as follows:
[0059]
[0060] (1) Synthesis of 1,3-diglyceride (1,3-DG): 2.5g (12.48mmol) lauric acid was dissolved in 50ml of dichloromethane, in 3.7g (19.30mmol) EDCI and 0.7g (5.73 Under the catalysis of mmol) DMAP, react with 0.46g (5.11mmol) 1,3-dihydroxyacetone to form an ester, and then react with 0.2g (5.28mmol) sodium borohydride for hydrogenation to obtain 1,3-DG.
[0061] (2) Dissolve 0.7g (2.03mmol) of 4,4'-dithiodibutyric acid in 4ml of acetic anhydride, react at room temperature for 2h, concentrate by rotary evaporation and redissolve in 30ml of anhydrous dichloromethane, And added 0.89g (1.83mmol) 1,3-DG, 28.9mg (0.29mmol) DMAP. Vacuum nitrogen protection at room temperature for 12h. The solvent was removed by rotary evaporation, and the intermediate product was obtained ...
Embodiment 3
[0068] The preparation method of the SN38 class triglyceride prodrug (SN38-SS-PA) substituted by palmitic acid group comprises the following steps:
[0069] The structure of the prodrug is as follows:
[0070]
[0071] (1) Synthesis of 1,3-diglyceride (1,3-DG): 2.5g (9.76mmol) of palmitic acid was dissolved in 50ml of dichloromethane, and 2.4g (12.06mmol) of EDCI and 0.5g (4.10 mmol) DMAP catalyzed with 0.3g (3.33mmol) 1,3-dihydroxyacetone to form an ester reaction, and then hydrogenated with 0.2g (5.28mmol) sodium borohydride to obtain 1,3-DG.
[0072](2) Dissolve 0.7g (2.03mmol) of 4,4'-dithiodibutyric acid in 4ml of acetic anhydride, react at room temperature for 2h, concentrate by rotary evaporation and redissolve in 30ml of anhydrous dichloromethane, And 1.09 g (1.83 mmol) 1,3-DG, 35.6 mg (0.29 mmol) DMAP were added. Vacuum nitrogen protection at room temperature for 12h. The solvent was removed by rotary evaporation, and the intermediate product was obtained by col...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com