Intravenous injection liquid of coenzyme Q10, and its prepn. method
A technology of intravenous infusion and coenzyme, applied in drug delivery, blood diseases, pharmaceutical formulations, etc., can solve the problems of low bioavailability, environment, equipment pollution, user infusion reaction, etc., to improve bioavailability and avoid crossover Effect of infecting and widening the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The following specific embodiments are further descriptions of the present invention, and the present invention is not limited to the scope of the described embodiments.
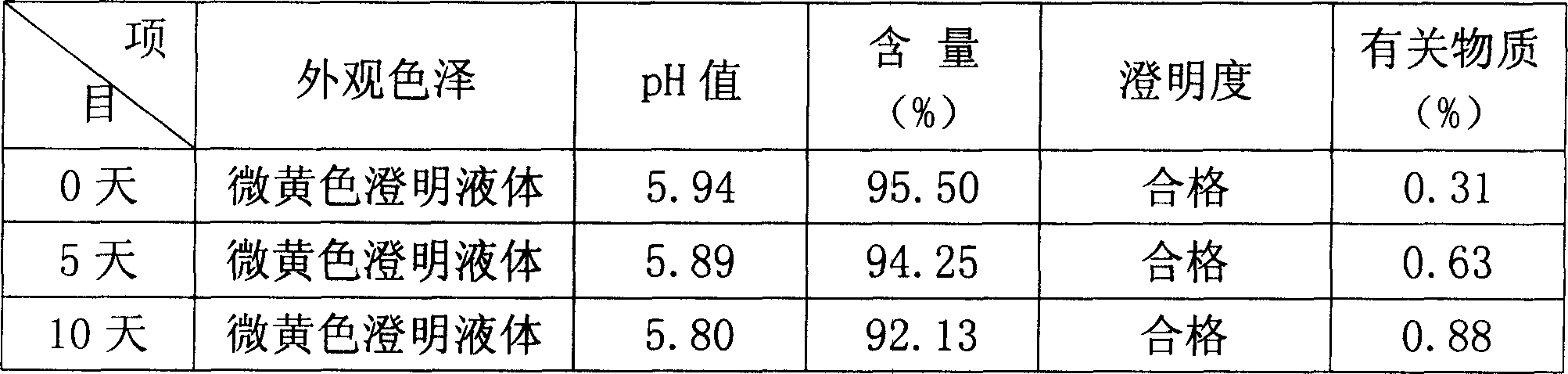
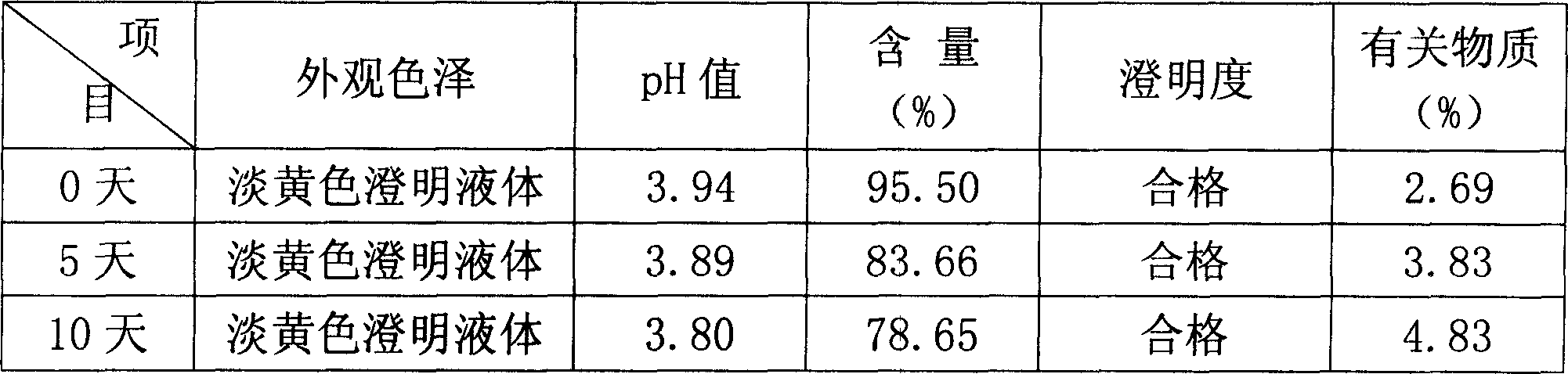
[0025] In the following examples, the preparation specification of the product formula is 5-60 mg / bottle, wherein coenzyme Q 10 The main drug is administered separately according to the formula according to different specification requirements, and the pH value of the solution is in the range of 4.0-7.0; the solvent for injection is used as the solvent for dissolving raw materials, and an appropriate amount is added according to the concentration of the main drug and the filling amount of each drug.
[0026] Numbering
Main drug
Solubilizers
solvent for injection
osmotic regulator
Total (bottle)
1
coenzyme Q 10
5-60g
Polysorbate 80:30g
Add water for injection to
100L
Sodium chloride
0.6kg
1000 bottle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com