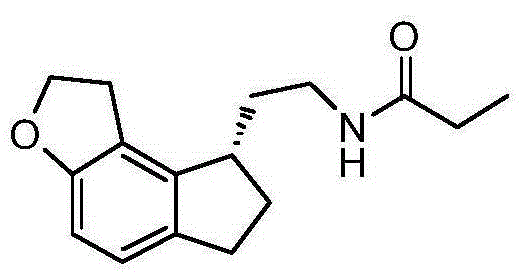
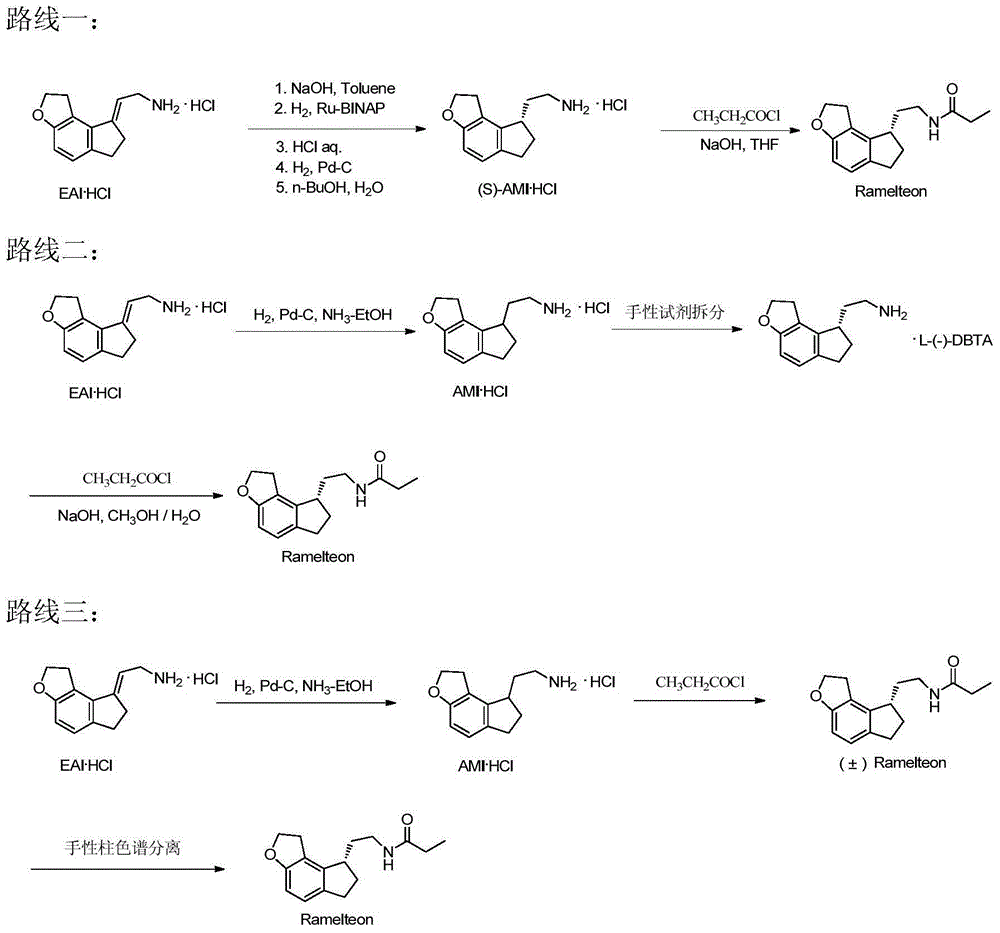
Preparation method of Ramelteon
A technology of ramelteon and a purification method, which is applied in the field of preparation of ramelteon, can solve the problems of expensive chiral catalytic reagents, harsh hydrogenation operation requirements, and high requirements for operators, and achieves suitable large-scale industrial production, catalysis and catalysis. Good effect, stable process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
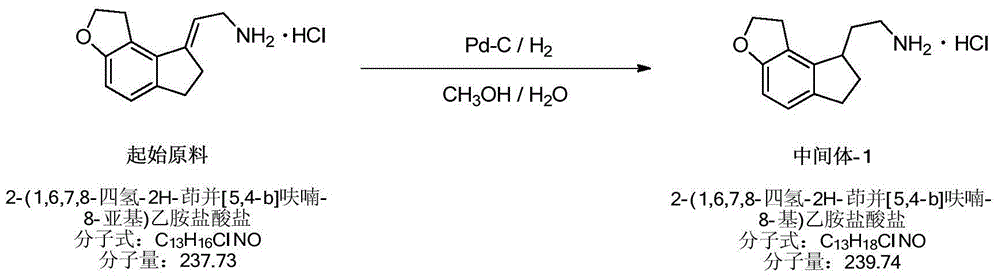
[0053] Hydrogenation reaction:
[0054] The material proportion of hydrogenation reaction:
[0055] Table 1 hydrogenation reaction material ratio
[0056]
[0057] Hydrogenation operation process:
[0058] Weigh the starting materials 2-(1,6,7,8-tetrahydro-2H-indeno[5,4-b]furan-8-ylidene)ethylamine hydrochloride, 10%Pd- C and methanol-water, stirred evenly, vacuum pumped into the hydrogenation reaction kettle, vacuumed, added hydrogen, and stirred at room temperature for reaction.
[0059] When the hydrogen pressure no longer drops, the reaction is terminated, the pressure is released, the material is discharged, and the filter is suctioned. The filtrate is concentrated, and the solid is dried by blowing to obtain a light yellow solid, namely Intermediate-1.
[0060] Internal control standard for hydrogenation reaction:
[0061] Table 2 Intermediate-1 quality standard
[0062]
[0063] Chiral resolution:
[0064] Chiral resolution material ratio:
[0065] Table 3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com